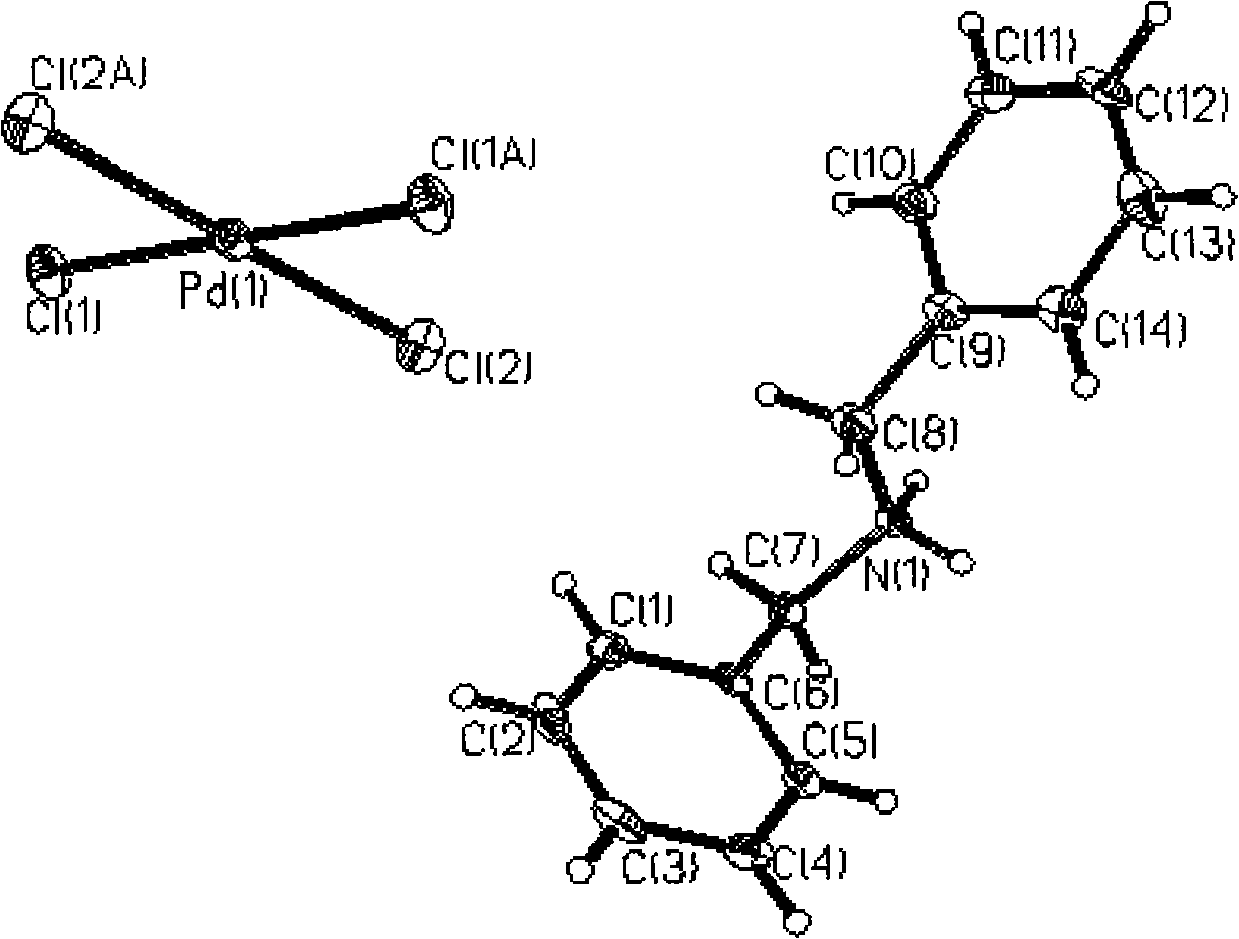
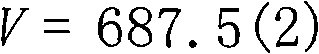Method for separating enriched palladium by adopting supermolecule secondary spherical coordination identification
A separation, enrichment and supramolecular technology, applied in the field of extracting metal palladium, can solve the problems of consuming oxidants and reducing agents, and achieve the effect of broad industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of protonated dibenzylamine
[0019] Take 5g of dibenzylamine, add 10ml of chloroform, after dissolving, add 0.5ml of 37% hydrochloric acid, a white precipitate is formed, filter and dry in vacuo.
[0020] (2) Preparation of palladium-containing sample solution
[0021] PdCl 2 0.0201g, AlCl 3 0.0153g, FeCl 3 ·6H 2 O 0.0316g, NiCl 2 ·6H 2 O 0.0256g, CoCl 2 ·6H 2 O 0.0269g, CuCl 2 2H 2 O 0.0185g, MnCl 2 4H 2 O 0.0229g, add to 150mL beaker, add 10ml of water and 1ml of concentrated hydrochloric acid, dissolve.
[0022] (3) Separation and enrichment of palladium:
[0023] Add protonated dibenzylamine methanol solution (protonated dibenzylamine 0.15g, dissolved in 20ml methanol) to the palladium-containing sample solution prepared above, add 1ml 20% hydrochloric acid, let stand for 20 hours, and precipitate reddish-brown crystals. After filtration, the obtained solid was vacuum-dried to obtain complex powder, and the recovery rate of palladium...
Embodiment 2
[0034] (1) Preparation of protonated dibenzylamine
[0035] Take 5g of dibenzylamine, add 10ml of chloroform, after dissolving, add 0.5ml of 37% hydrochloric acid, a white precipitate is formed, filter and dry in vacuo.
[0036] (2) Preparation of palladium-containing sample solution
[0037] As shown in Table 2, weigh PdCl 2 and AlCl 3 In a 150mL beaker, add 5ml of water and 0.5ml of hydrochloric acid.
[0038] (3) Separation and enrichment of palladium:
[0039] Add an ethanol solution of protonated dibenzylamine to the palladium-containing sample solution prepared above (0.1 g of protonated dibenzylamine is dissolved in 20 ml of ethanol), add 1 ml of 10% sulfuric acid, and let it stand for 15 hours to obtain a reddish-brown solution, and a reddish-brown solution is precipitated. brown crystals. Filter and dry in vacuo to obtain complex powder.
[0040] Take 0.1975g of the dried complex powder, add 10ml of ammonia water, stir in a closed container for 50min until the r...
Embodiment 3
[0046] (1) Preparation of protonated dibenzylamine
[0047] Take 5g of dibenzylamine, add 10ml of chloroform, after dissolving, add 0.5ml of 37% hydrochloric acid, a white precipitate is formed, filter and dry in vacuo.
[0048] (2) Preparation of palladium-containing sample solution
[0049] As shown in Table 3, weigh PdCl 2 and FeCl 3 ·6H 2 O was dissolved in a 150mL beaker by adding 5ml of water and 0.5ml of hydrochloric acid.
[0050] (3) Separation and enrichment of palladium:
[0051] Add protonated dibenzylamine tert-butanol solution (protonated dibenzylamine 0.10g, dissolve in 20ml tert-butanol) to the palladium-containing sample solution prepared above, add 1ml 12% nitric acid, and let it stand for 24 hours to obtain Reddish-brown solution, reddish-brown crystals were precipitated. Filter and dry in vacuo to obtain complex powder.
[0052] Take 0.2012g of the dried complex powder, add 10ml of ammonia water, stir in a closed container for 50min until the reddish...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com