Surface self-assembly gold nanoprobe with free radical capture performance and preparing method and application thereof
A technology that captures free radicals and free radicals. It is used in medical preparations containing active ingredients, organic active ingredients, and drug combinations. It can solve the problems of limited capture performance, slow speed, and cell apoptosis, and achieve efficient and rapid capture and acceleration. Reaction speed, effect of enhancing chemical reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1. Method 1 Preparation of surface self-assembled gold nanoprobe B with high-efficiency free radical capture performance, R=NH, n=2.
[0071] Preparation of compound 5 (CMPO)
[0072]
[0073] Preparation of compound 1
[0074] N 2 Under protection, 7.5 mL of ethanol, 7.6 mL of pyridine, and 50 mL of chloroform were added to the single-necked flask. Under an ice-water bath, 15 mL of chloroform and 9.9 mL of 2-bromopropionyl bromide were slowly added dropwise. React overnight at room temperature to obtain a light yellow solution. After the reaction is complete, respectively, with 10% H 2 SO 4 solution, saturated NaHCO 3 Solution washing, anhydrous MgSO 4 dry. After filtration, the chloroform was removed by rotary evaporation to obtain 14 g of the product, and 8 g of the product were obtained after vacuum distillation (b.p=43-45° C. 0.05 mmHg). Yield 49%.
[0075] Preparation of compound 2
[0076] Add 10.8g NaNO to the single-necked bottle 2 , 12.8...
Embodiment 2
[0091] Example 2. Preparation of surface self-assembled gold nanoprobe B (R=0, n=11) with high-efficiency free radical capture performance
[0092] 1. The preparation process of tetraoctylammonium bromide-protected gold nanoparticles is as in Example 1.
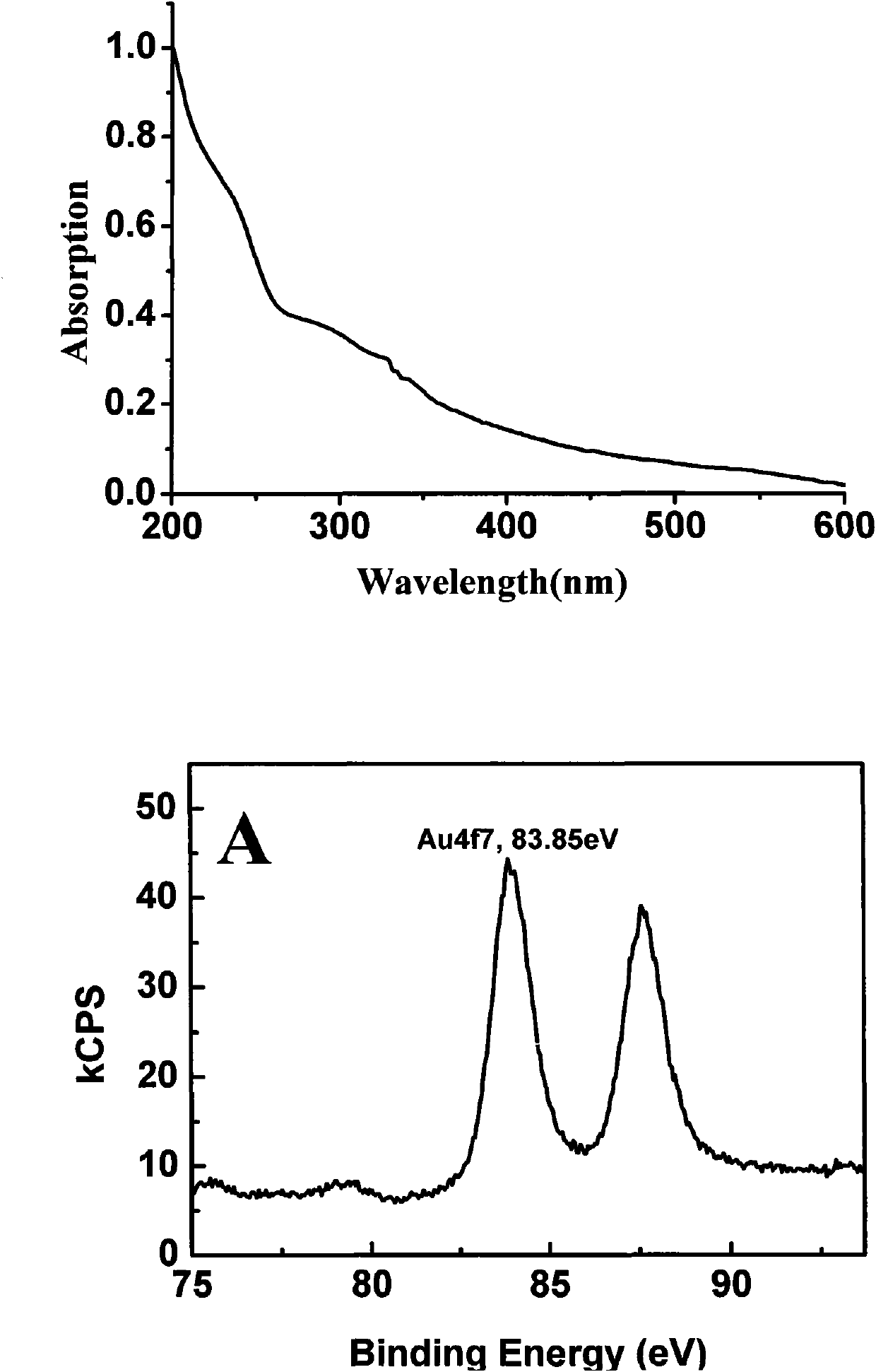
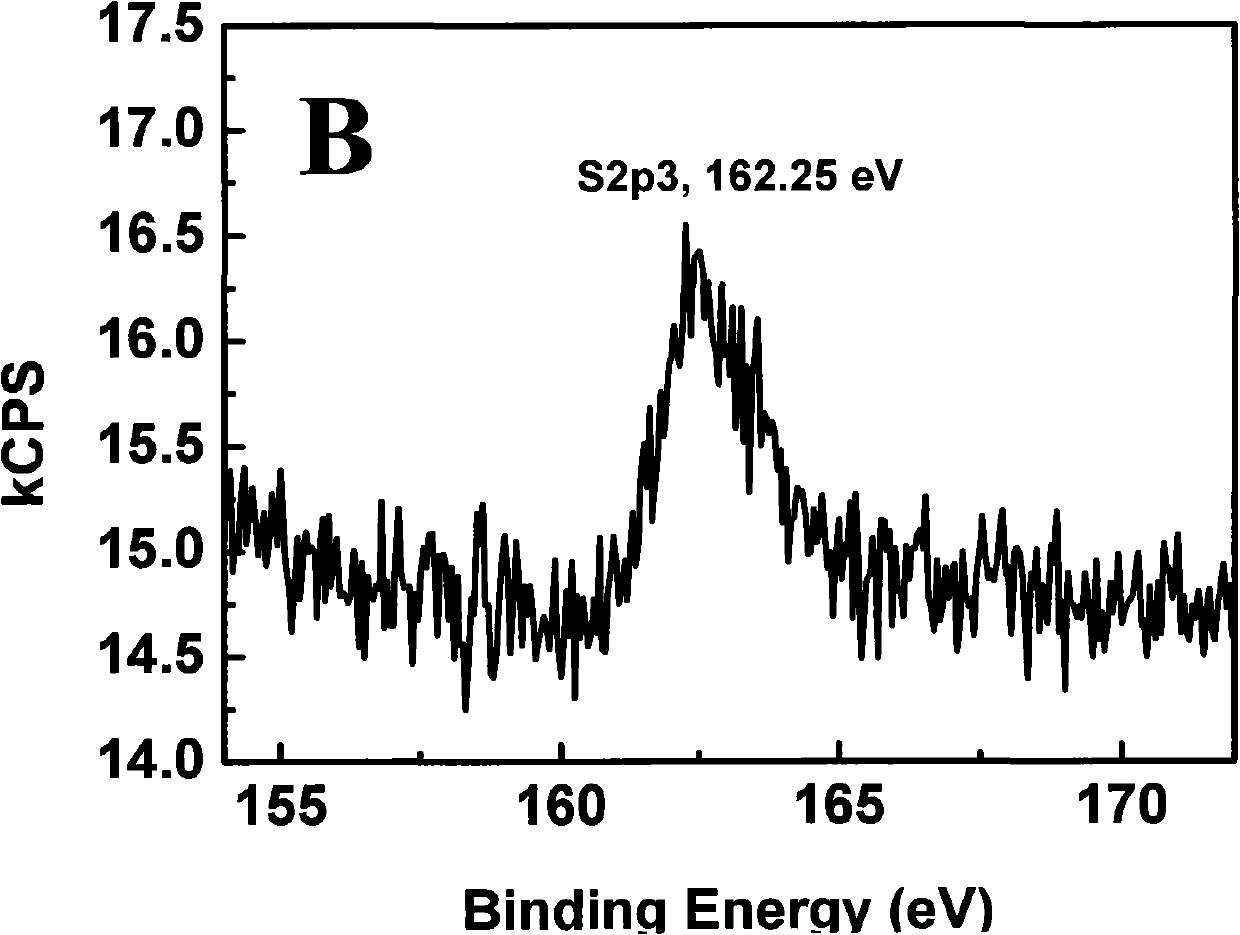
[0093] 2. Take 10 mg of tetraoctyl ammonium bromide-protected gold nanoparticles and 8.6 mg of ω-hydroxyundecyl mercaptan dissolved in an appropriate amount of solvent, and stir at room temperature for 6-24 hours. After the reaction was completed, the solvent was distilled off, and the obtained residue was dissolved in methanol, and purified by Sephadex LH-20 to obtain purified gold nanoparticles C (R=O, n=11). The characteristic absorption peak of its infrared spectrogram has a hydroxyl absorption peak at 3358; XPS results show that the ratio of gold atoms to sulfur atoms in gold nanoparticles C (R=O, n=11) is 4.5: 1; TEM results show that the average particle size The diameter is 4.5±0.5nm.
[0094] 3. Take 20 mg of gold ...
Embodiment 3
[0096] Example 3. Preparation of surface self-assembled gold nanoprobe B (R=O, n=4) with high-efficiency free radical capture performance
[0097] 1. The preparation process of tetraoctylammonium bromide-protected gold nanoparticles is as in Example 1.
[0098] 2. Take 55mg (2.4×10 -4 mol) ω-aminobutyl disulfide hydrochloride, 72mg (5×10 -4 mol) CMPO, 73.7mg (6.0×10 -4 mol) DMAP, 81.6mg (6.0×10 -4 mol)HOBt, 267.2mg (6.0×10 -4 mol) BOP, 400-800μl (2.0-4.0×10 -3 mol) NMM, dissolved in an appropriate amount of solvent, reacted at room temperature for 12-24 hours, after removing the solvent, with an appropriate amount of CH 2 Cl 2 After dissolution, respectively, with HCl solution, saturated NaHCO 3 solution, washed with saturated NaCl solution, anhydrous Na 2 SO 4 dry. Column separation yielded 60 mg of the product. 1 H-NMR (400MHz, CHCl 3 )δ: 1.33(s, 6H), 1.40-1.56(m, 16H), 2.56(t, 4H), 3.20(t, 4H), 7.35(t, 2H), 8.10(m, 2H).
[0099] 3. Dissolve 10 mg of tetraoctyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com