Method for preparing ciprofloxacin hydrochloride time-release capsule
A technology of ciprofloxacin hydrochloride and sustained-release capsules is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulas, urinary system diseases, etc., to achieve the effects of avoiding sudden release, smoothing the shape of the time curve and slowing the absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
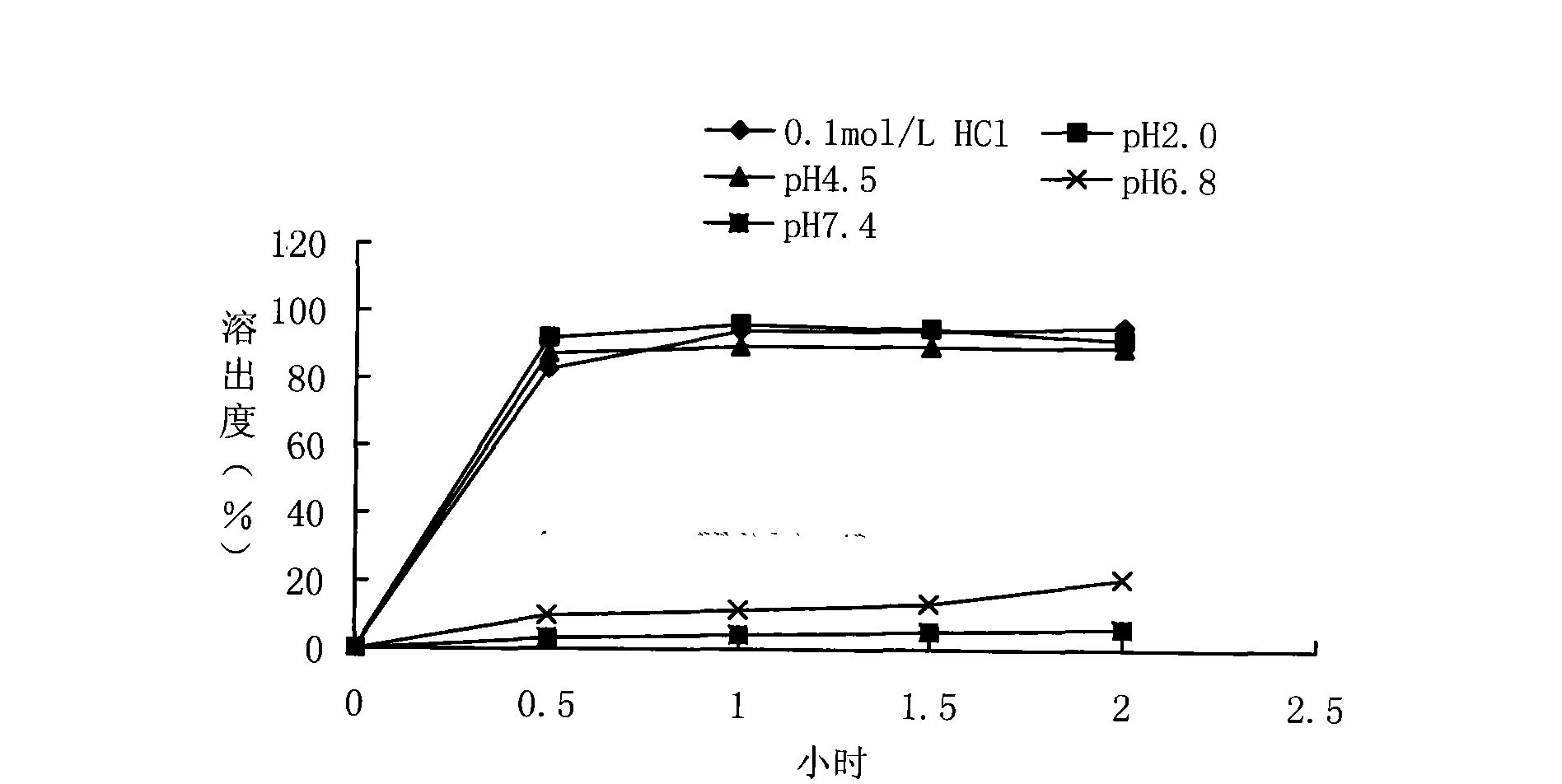
Embodiment 1
[0053] Take the production of 1000 ciprofloxacin hydrochloride sustained-release capsule products as an example, and its preparation method is as follows:
[0054] 1. Ingredients
[0055] Weigh the medicinal active ingredients and auxiliary materials according to the following mass:
[0056] Ciprofloxacin Hydrochloride 292g
[0057] Microcrystalline Cellulose 58.4g
[0058] Lactose 58.4g
[0059] Citric acid 8.3g
[0060] The mass ratio of the medicinal active ingredients and auxiliary materials of the above-mentioned medicine is:
[0061] Ciprofloxacin Hydrochloride 70%
[0062] Microcrystalline Cellulose 14%
[0063] Lactose 14%
[0064] Citric acid 2%
[0065] 2. mix
[0066] Grind ciprofloxacin hydrochloride through a 120-mesh sieve, crush lactose through an 80-mesh sieve, and ciprofloxacin hydrochloride through an 80-mesh sieve, weigh ciprofloxacin hydrochloride, lactose, and citric acid according to the above mass ratio and mix them with a mixer Evenly, prepare a...
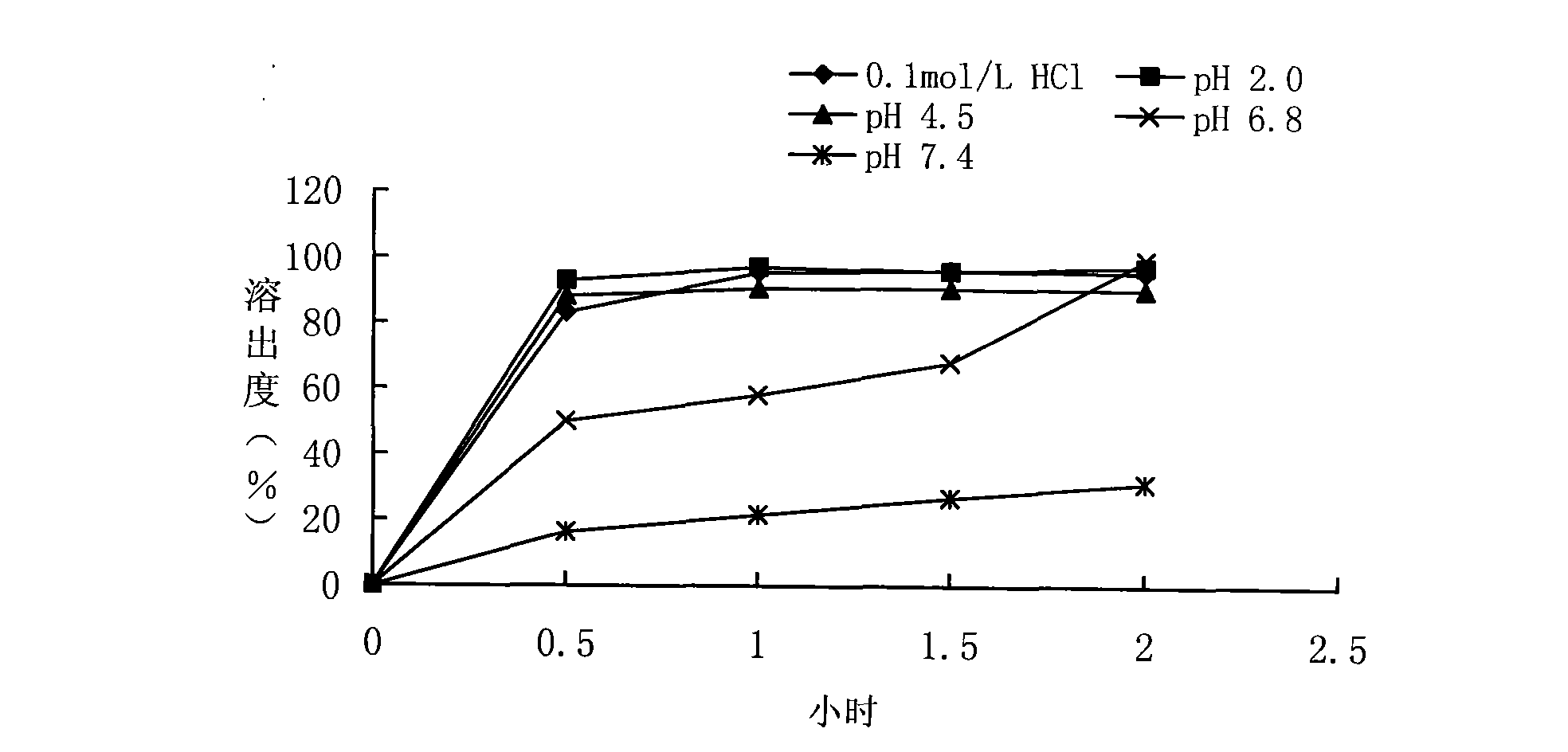
Embodiment 2
[0091] Take the production of 1000 ciprofloxacin hydrochloride sustained-release capsule products as an example, and its preparation method is as follows:
[0092] In the step 3 of preparing the plain pill, the polishing time is 3 minutes, and the other steps of this step are the same as in Example 1. In the coating step 4, when the coating amount of 1000g of plain pills is 1.2%, the coating solution is made from the raw materials of the following mass ratio:
[0093] Aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer 14.26g
[0094] Aqueous dispersion of methacrylic acid-ethyl acrylate copolymer 14.26g
[0096] Polyethylene glycol 6000 1.78g
[0097] Sodium Lauryl Sulfate 1.43g
[0098] Water 317.64mL
[0099] The mass percent of coating solution is:
[0100] Aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer 4%
[0101] Aqueous dispersion of methacrylic acid-ethyl acrylate copolymer 4%
[0102] Talc 2%
[01...
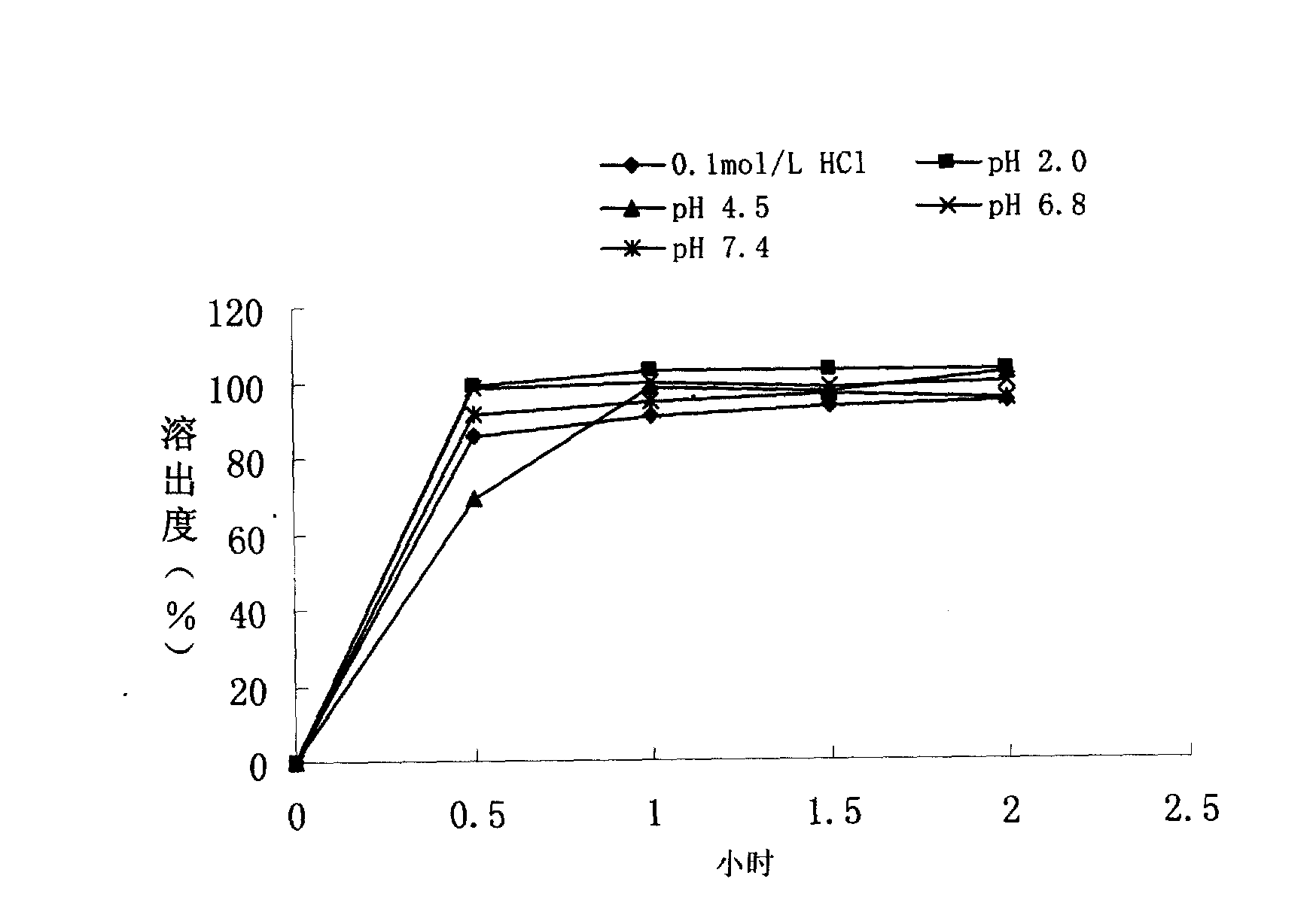
Embodiment 3
[0108] Take the production of 1000 ciprofloxacin hydrochloride sustained-release capsule products as an example, and its preparation method is as follows:
[0109] In the step 3 of preparing the plain pill, the polishing time is 4 minutes, and the other steps of this step are the same as in Example 1. In the coating step 4, when the coating amount of 1000g of plain pills is 1.2%, the coating solution is made from the raw materials of the following mass ratio:
[0110] Aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer 24.96g
[0111] Aqueous dispersion of methacrylic acid-ethyl acrylate copolymer 24.96g
[0113] Polyethylene glycol 6000 2.85g
[0114] Sodium Lauryl Sulfate 2.50g
[0115] Water 283.40mL
[0116] The mass percent of coating solution is:
[0117] Aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer 7%
[0118] Aqueous dispersion of methacrylic acid-ethyl acrylate copolymer 7%
[0119] Talc 5%
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com