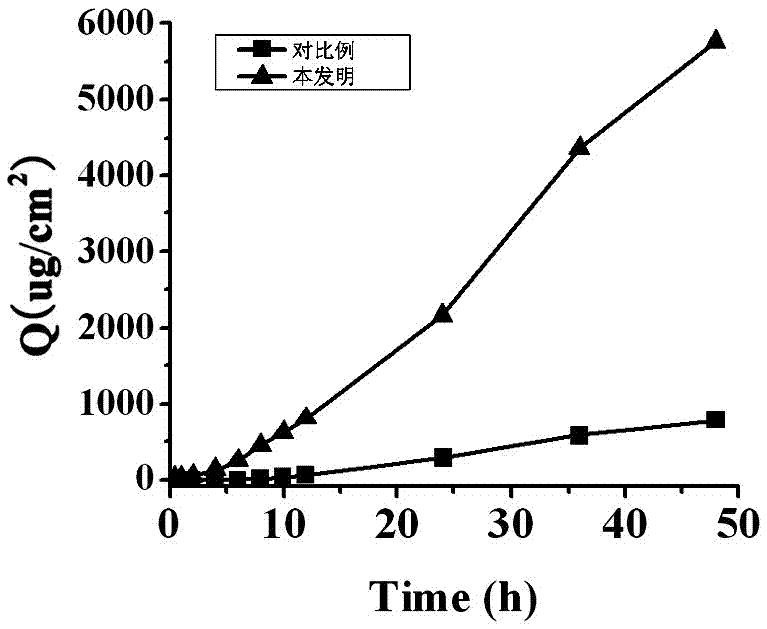
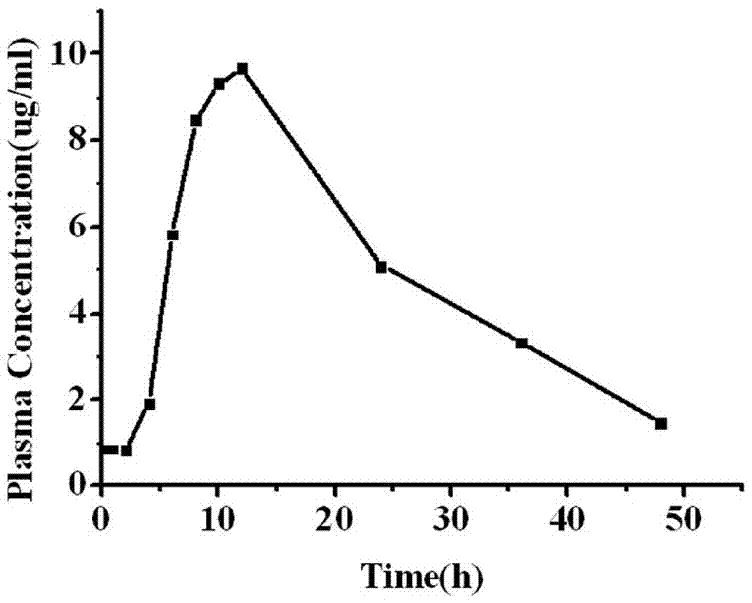
A kind of glimepiride hydrogel and preparation method thereof
A technology of hydrogel and glimepiride, which is applied in the field of glimepiride hydrogel and its preparation, can solve the problems of limited oral drug dissolution, difficult control of drug dosage, and poor biocompatibility. Achieve the effects of avoiding the first pass effect of the liver, lasting blood drug concentration, and high drug dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 100 mg of glimepiride and 400 mg of meglumine co-solvent, heat and dissolve with 5 g of purified water in a water bath at 80°C, stir and let cool to obtain 20 mg / ml glimepiride co-solvent aqueous solution; Add 0.0456g of azone and 0.0446g of oleic acid respectively in this solution, after magnetic stirring evenly, add the ethanol solution of 10% (g / mL) ethylparaben (containing 0.01g ethylparaben) successively , 0.001g EDTA-2Na and 0.652g glycerin, after stirring evenly, it becomes a clear solution, add 50mg carbomer, stir magnetically, make it fully swell and form a gel, add 0.0672g triethanolamine, stir overnight, and obtain a transparent lattice Limepiride hypoglycemic hydrogel.
Embodiment 2
[0026] Weigh 125 mg of glimepiride and 500 mg of meglumine co-solvent, heat and dissolve with 5.04 g of purified water in a water bath at 90°C, stir and let cool to obtain an aqueous solution of glimepiride co-solvent; add 0.107 g of co-solvent to the solution Azone and 0.107g of oleic acid, after magnetic stirring evenly, add 8% (g / mL) ethylparaben ethanol solution (containing 0.018g ethylparaben), 0.0018g EDTA-2Na and 1.071 g glycerin, after stirring evenly, it becomes a clear solution, add 71.4mg carbomer, stir magnetically, make it fully swell and form a gel, add 0.1071g triethanolamine, stir overnight, and obtain transparent glimepiride hypoglycemic hydrogel Glue.
Embodiment 3
[0028]Weigh 75 mg of glimepiride and 300 mg of meglumine co-solvent, heat and dissolve with 4.87 g of purified water in a water bath at 60°C, stir and let cool to obtain an aqueous solution of glimepiride co-solvent; add 0.03 g of co-solvent to the solution Azone and 0.03g of oleic acid, magnetically stirred evenly, then added 5% (g / mL) ethylparaben ethanol solution (containing 0.0006g ethylparaben), 0.061mg EDTA-2Na and 0.60 g glycerin, after stirring evenly, it becomes a clear solution, add 30mg carbomer, stir magnetically, make it fully swell and form a gel, add 0.060g triethanolamine, stir overnight, and obtain transparent glimepiride hypoglycemic hydrogel agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com