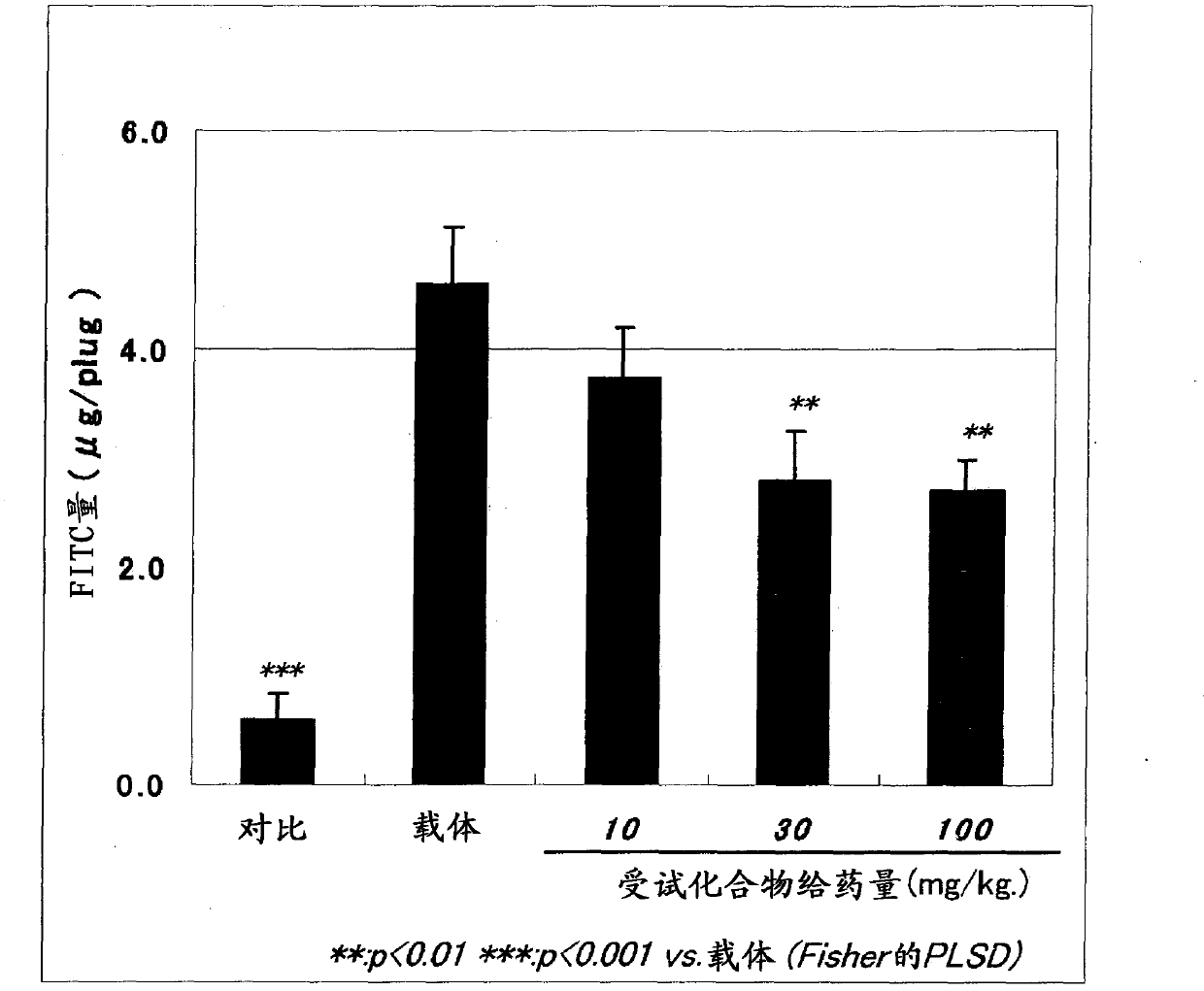
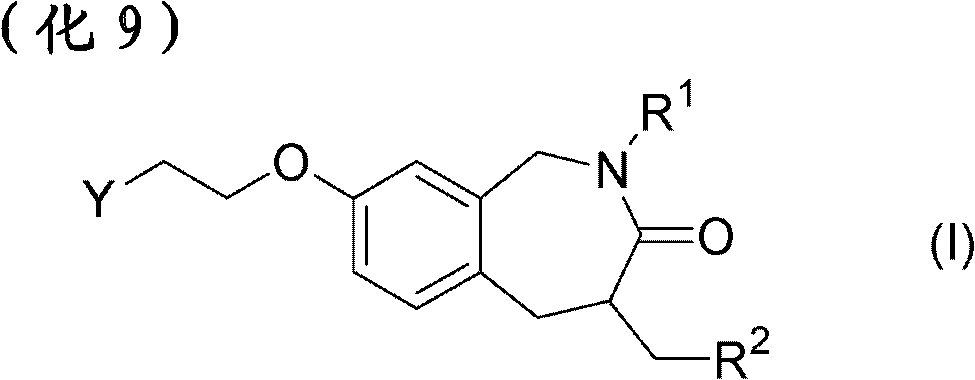
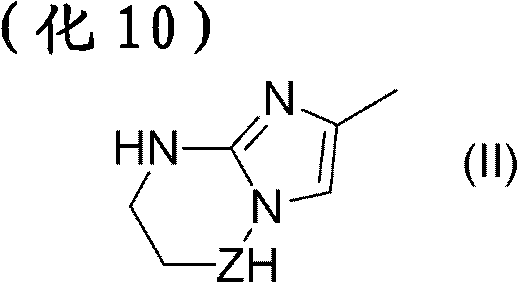
Pharmaceutical composition for treatment or prevention of ophthalmic diseases
A technology for compositions and medicines, applied in the directions of medicine combinations, active ingredients of heterocyclic compounds, sensory diseases, etc., can solve problems such as ketone compounds that are not specifically disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0396] 3-Oxo-8-[2-(5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-2-yl)ethoxy]-2-(2,2,2- Trifluoroethyl)-2,3,4,5-tetrahydro-1H-2-benzazepine -4-Ethyl acetate hydrochloride
[0397] 1-(a)1-Bromo-4-hydroxy-2-butanone
[0398] To a solution of 169 g (1.92 mol) of 4-hydroxy-2-butanone in 1.9 L of methanol was added dropwise 300 g (1.88 mol) of bromine at -5°C over 30 minutes. After the dropwise addition, the temperature was slowly raised to room temperature, and stirred for 2 hours. Next, 3.84 L (3.84 mol) of 2N sulfuric acid was added at 0°C, stirred at 10°C for 3.5 hours, and further stirred at room temperature for 22 hours.
[0399] After completion of the reaction, 325 g of sodium chloride was added to the reaction solution, followed by extraction with 5.4 L of a mixed solution of chloroform:methanol=2:1 (V / V). Furthermore, the aqueous layer was extracted four times with 1.7 L of a mixed solution of chloroform:methanol=9:1 (V / V). The combined organic layers were washed with s...
Embodiment 2
[0418] 3-Oxo-8-[2-(5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-2-yl)ethoxy]-2-(2,2,2- Trifluoroethyl)-2,3,4,5-tetrahydro-1H-2-benzazepine -4-Acetic acid trifluoroacetate
[0419] The 3-oxo-8-[2-(5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-2-yl)ethoxy obtained in Example 1-(d) ]-2-(2,2,2-trifluoroethyl)-2,3,4,5-tetrahydro-1H-2-benzazepine - 0.054 g (0.10 mmol) of 4-ethyl acetate hydrochloride was dissolved in 2 mL of methanol: tetrahydrofuran = 1: 1 mixed solution, and 0.2 mL of water and 0.3 mL of 1 equivalent of aqueous sodium hydroxide solution were added under ice-cooling. Stir for 2 hours.
[0420] After the reaction, 0.025 mL of acetic acid was added to the reaction solution, and concentrated under reduced pressure. The obtained residue was subjected to solid-phase extraction (cartridge: Sep-Pak C18 (5 g / 20 mL (manufactured by Waters), elution solvent: 0.05% trifluoroacetic acid aqueous solution → acetonitrile: 0.05% trifluoroacetic acid aqueous solution = 3: 7 (V / V)) ...
Embodiment 3
[0424] 3-Oxo-8-[2-(1,2,3,4-tetrahydroimidazo[1,2-b][1,2,4]triazin-6-yl)ethoxy]-2 -(2,2,2-Trifluoroethyl)-2,3,4,5-tetrahydro-1H-2-benzazepine -4-Acetic acid trifluoroacetate
[0425] 3-(a) 2-(imidazo[1,2-b][1,2,4]triazin-6-yl)ethanol
[0426] To a solution of 1.92 g (20.0 mmol) of 3-amino-1,2,4-triazine in 40 mL of ethanol, 3.67 g of 1-bromo-4-hydroxyl-2-butanone obtained in Example 1-(a) was added g (22.0mmol), heated to reflux for 2 hours.
[0427] After completion of the reaction, the reaction solution was concentrated under reduced pressure, 200 mL of a mixed solution of chloroform:methanol=9:1 (V / V) was added to the obtained residue, and insoluble matter was removed by filtration. The filtrate was washed successively with water and saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting residue was subjected to silica gel column chromatography (elution solvent: chloroform:methanol=30:1→9:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com