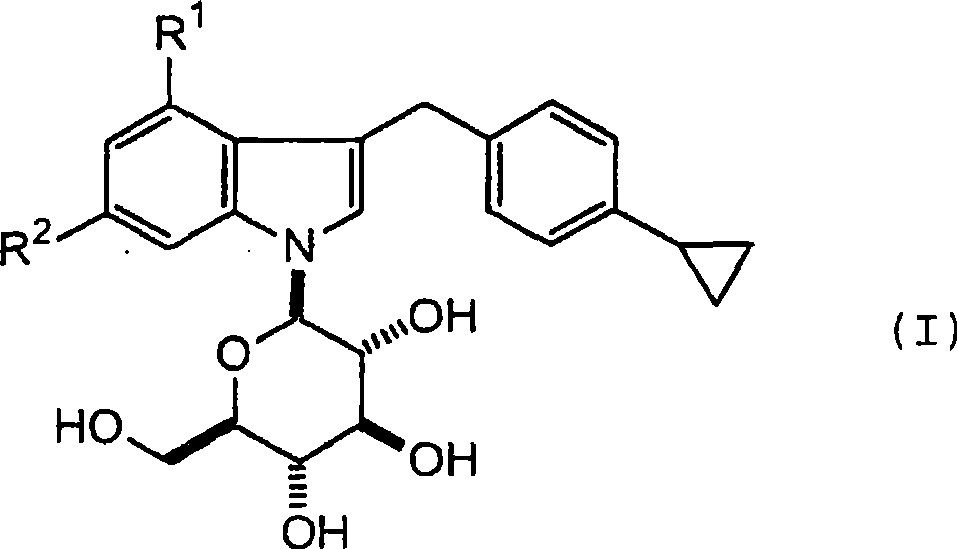
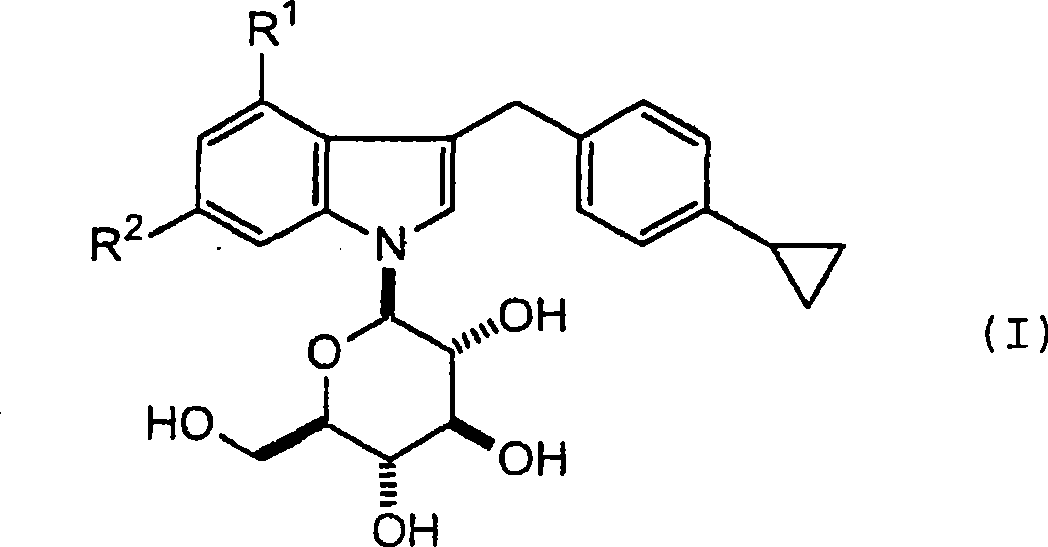
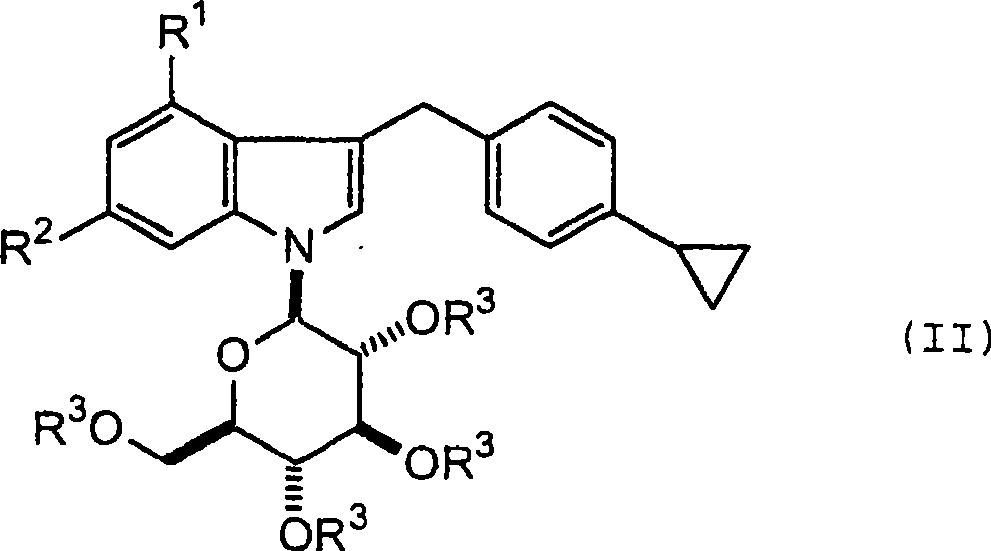
1- (-d-glycopyranosyl) - 3 - (4-cyclopropylphenylmethyl) - 4 - halogeno indole derivatives and use thereof as sglt inhibitors
A technology of cyclopropylphenylmethyl and glucopyranosyl, applied in the field of new indole derivatives, can solve problems such as lactic acidosis, hypoglycemia, edema and heart failure, achieve excellent results, and excellently lower blood sugar Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] 3-(4-Cyclopropylphenylmethyl)-4-fluoro-1-(β-D-glucopyranosyl)indole
[0135]
[0136] (1) A mixture of 4-fluoroindoline (185mg) and D-glucose (267mg) in water (0.74ml)-ethanol (9ml) was refluxed under argon for 24 hours. The solvent was evaporated under reduced pressure to give crude 4-fluoro-1-(β-D-glucopyranosyl)indoline, which was used in the next step without further purification.
[0137](2) The above compound was suspended in chloroform (8ml), and pyridine (0.873ml), acetic anhydride (1.02ml) and 4-(dimethylamino)pyridine (catalytic amount) were sequentially added thereto. After stirring at room temperature for 21 hours, the reaction solvent was evaporated under reduced pressure. The residue was dissolved in ethyl acetate, and the solution was washed twice with 10% aqueous copper(II) sulfate, then with saturated aqueous sodium bicarbonate, and dried over magnesium sulfate. The insoluble matter was filtered off, and the filtrate was evaporated under reduced pr...
Embodiment 2
[0143] Example 2: 4-Chloro-3-(4-cyclopropylphenyl-methyl)-1-(B-D-glucopyranosyl)indole
[0144]
[0145] (1) Take 4-chloroindoline (2.88g) and D-glucose (3.38g) in ethanol (150ml)-H 2 O (10 mL) the mixture was refluxed overnight under argon. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (chloroform:methanol=100:0-88:12) to obtain 4-chloro-1-(β-D-glucopyranosyl)indoline ( 3.35 g) of a colorless foam. APCI-mass spectrum m / Z 316 / 318 (M+H). 1 H-NMR (DMSO-d 6)δ 2.87-3.02(m, 2H), 3.07-3.12(m, 1H), 3.20-3.32(m, 2H), 3.38-3.47(m, 2H), 3.51-3.60(m, 2H), 3.68-3.73 (m, 1H), 4.34-4.37(m, 1H), 4.63(d, J=8.3Hz, 1H), 4.93(d, J=5.1Hz, 1H), 5.03(d, J=4.0Hz, 1H) , 5.06 (d, J=4.5Hz, 1H), 6.53 (d, J=8.0Hz, 1H), 6.60 (d, J=8.0Hz, 1H), 6.99 (t, J=7.9Hz, 1H).
[0146] (2) Dissolve the above-mentioned compound (3.3g) in 1,4-dioxane (150ml), and add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone ( 2.85g). The mixture was sti...
Embodiment 3
[0153] 3-(4-Cyclopropylphenylmethyl)-4,6-difluoro-1-(β-D-glucopyranosyl)indole
[0154]
[0155] In a similar manner to Example 1, the title compound was obtained as a colorless foam from 4,6-difluoroindoline. APCI-mass spectrum m / Z 463 (M+NH 4 ). 1 H-NMR (DMSO-d 6 )δ 0.58-0.62(m, 2H), 0.88-0.91(m, 2H), 1.82-1.88(m, 1H), 3.20-3.50(m, 4H), 3.59-3.70(m, 2H), 3.99(s , 2H), 4.54(t, J=5.7Hz, 1H), 5.10(d, J=5.3Hz, 1H), 5.19(d, J=5.0Hz, 1H), 5.22(d, J=5.8Hz, 1H ), 5.35(d, J=9.0Hz, 1H), 6.78(t, J=9.6Hz, 1H), 6.96(d, J=8.0Hz, 2H), 7.11(d, J=8.0Hz, 2H), 7.22 (s, 1H), 7.30 (dd, J=10.0, 1.7Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com