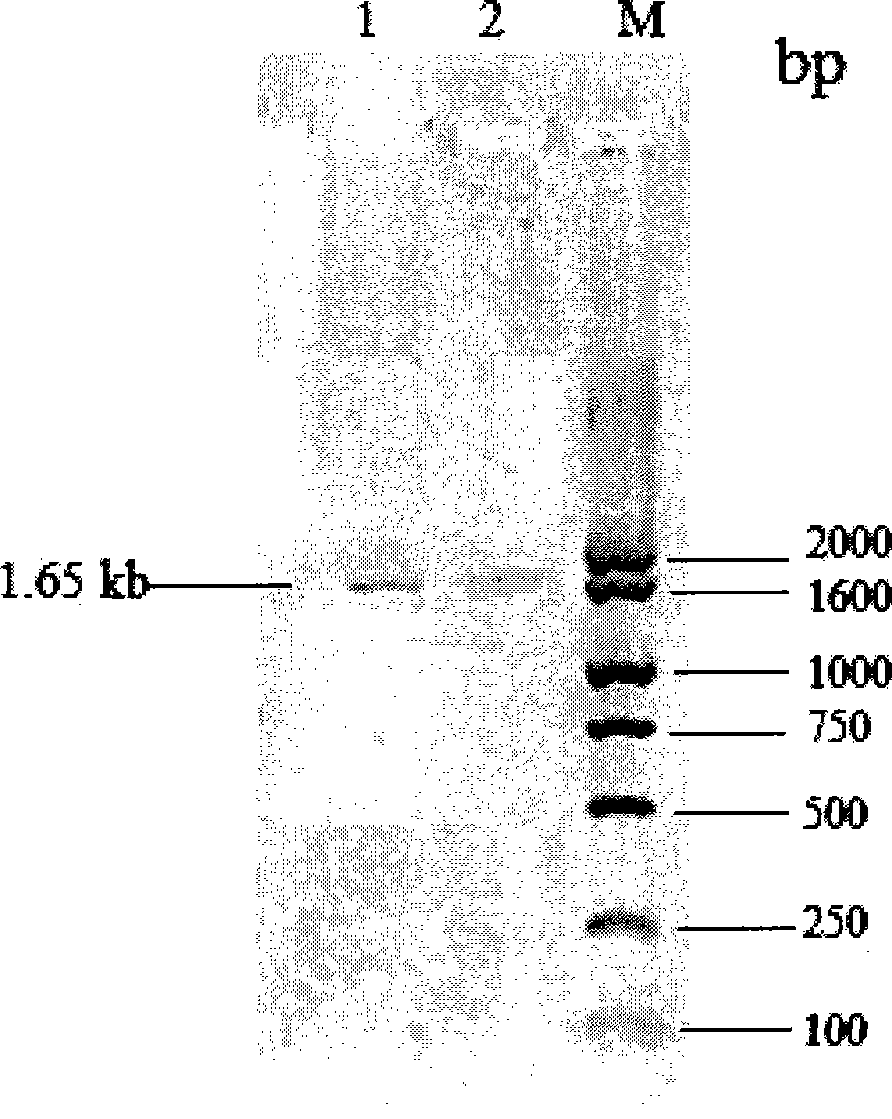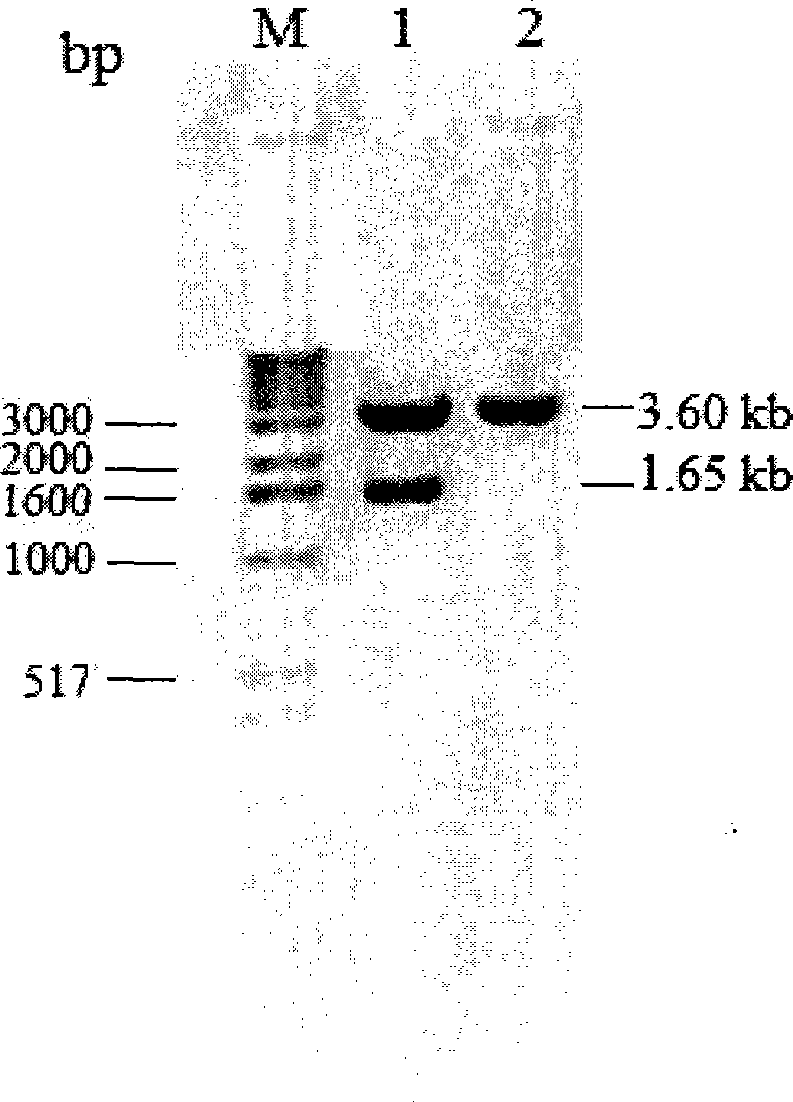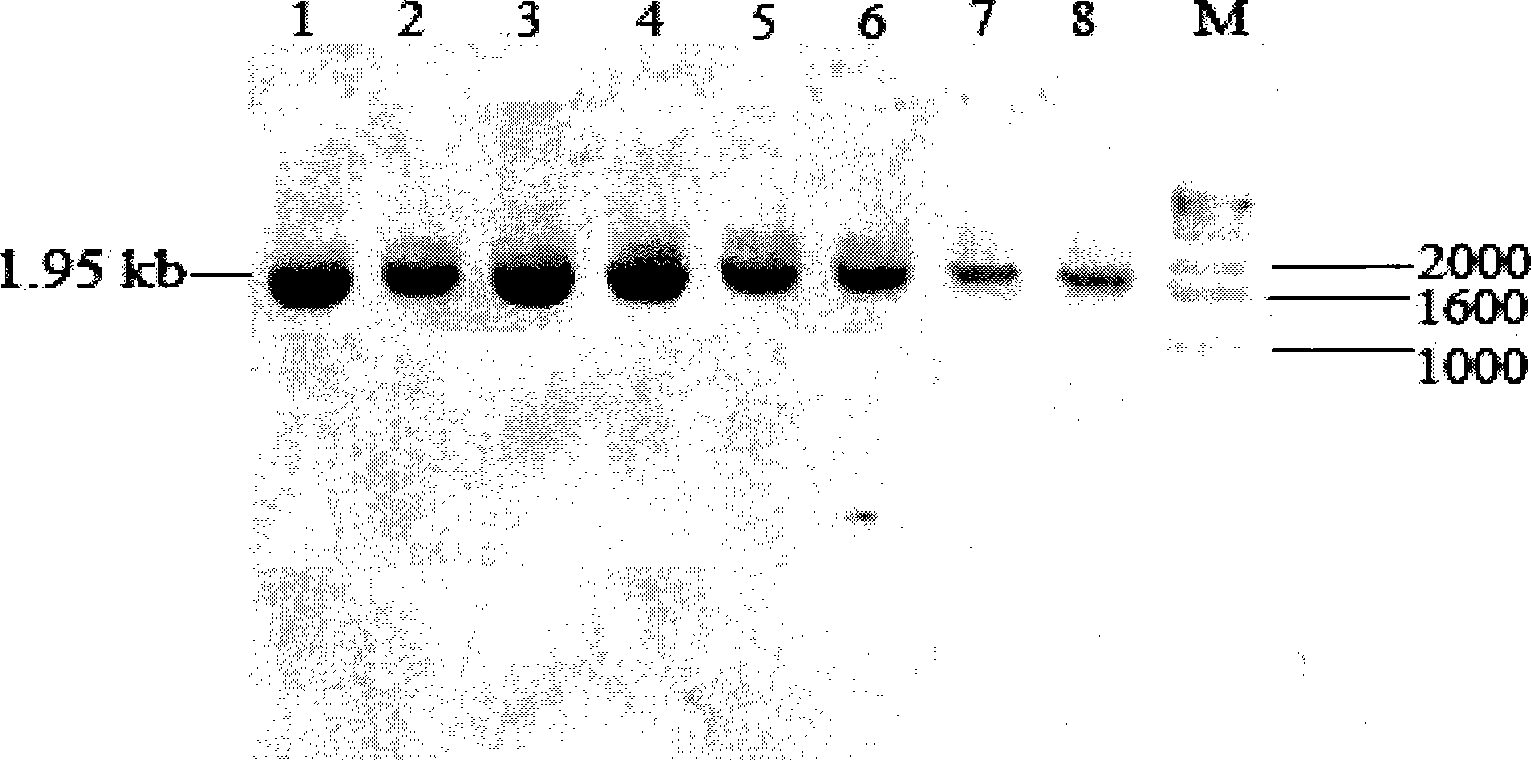Secretory expression method for exoinulinase from Kluyveromyces marxianus
A technology of exo-inulinase and expression method, which is applied in the field of expression of exo-inulinase, can solve the problems of inability to form natural inulinase molecules and affect the biological activity of recombinant inulinase, and achieve the goal of a good enzymatic tool Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Cloning and sequencing of the signal peptide inulinase gene of Kluyveromyces marxianus
[0029] The genomic DNA of K. marxianus CBS6556 was extracted according to the method described in "Experimental Guide to Molecular Biology" (Fourth Edition, Osber et al., translated by Yan Ziying et al., published by Science Press), V- 530 UV / Visible / Near Infrared Spectrometer (JASCO) to measure its OD 260 / OD 280 Value=1.76, frozen at -20°C for later use. Using the genomic DNA of K.marxianus CBS6556 as a template, using two specific primers inu-ORF-p1: 5'-ATGAAGTTAGCATACTCCCTCTTGC-3' and inu-ORF-p2: 5'-TCAAAGGTTAAATTGGGTAACGTT-3', according to conventional methods (molecular The third edition of the cloning experiment guide, written by Sam Brook, translated by Huang Peitang, etc., published by Science Press) for polymerase chain reaction (PCR). PCR system: 10×PCR buffer (Dalian TakaRa) 5.0μl, dNTPs (10mmol / l, TaKaRa) 1.0μl, inu-ORF-p1 primer (10mmol / l) 2.0μl, inu-ORF-p2 prim...
Embodiment 2
[0058] Example 2: Construction of recombinant Pichia pastoris expression vector pPICZαA-inu12
[0059] According to the sequence information of the two ends of the mature protein encoded by the K. marxianus CBS6556 inulinase gene and the sequence information of the polyclonal restriction site of the Pichia expression vector pPICZαA (purchased from Invitrogen), a pair of specific primers was designed. The sequence is as follows:
[0060] Inu-p1: 5’-TC CTC GATGGTGACAGCAAGGCCAT-3' (the underlined part is the XhoI digestion sequence, and the boxed part is the coding sequence of the Kex2 signal peptidase recognition site)
[0061] Inu-p2: 5’-CTC TCTAGA AAGGTTAAATTGGGTAACGTT-3' (the single underlined part is the Xba I restriction sequence, the double underlined part is the stop codon sequence)
[0062] Using the T vector carrying the K. marxianus CBS6556 inulinase gene obtained in Example 1 as a template, using primers Inu-p1 and Inu-p2, the encoding of the mature peptide of inulina...
Embodiment 3
[0120] Example 3: Transformation of Pichia pastoris strain and screening of positive clones
[0121] Recombinant expression plasmid inulinase was linearized with sac I (Dalian TakaRa) single enzyme digestion, after phenol: chloroform: isoamyl alcohol extraction to remove protein, add 1 / 10 volume of 3mol / l sodium acetate solution (pH 5.2) and 2 Double the volume of absolute ethanol, precipitate and recover the linearized vector. The purified linear vector is measured by V-530mol / l ultraviolet / visible light / near infrared spectrometer (JASCO) to have a concentration of 995ng / μl. Take 10 μl of the purified linear vector and transform the Pichia pastoris X-33 (purchased from Invitrogen) by the electric shock method, and transform the linearized pPICZαA empty vector as a control at the same time. Electric shock transformation parameters: Electric shock parameters: 0℃, 1.5kv, 200Ω, 25μF, electric shock time: 4.5-10ms. For the preparation of competent cells, please refer to the operation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com