Stable emulsion formulations
A technology of formulation and charge stabilizer, which is applied in the medical field and can solve the problems of drug stability and harmful effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
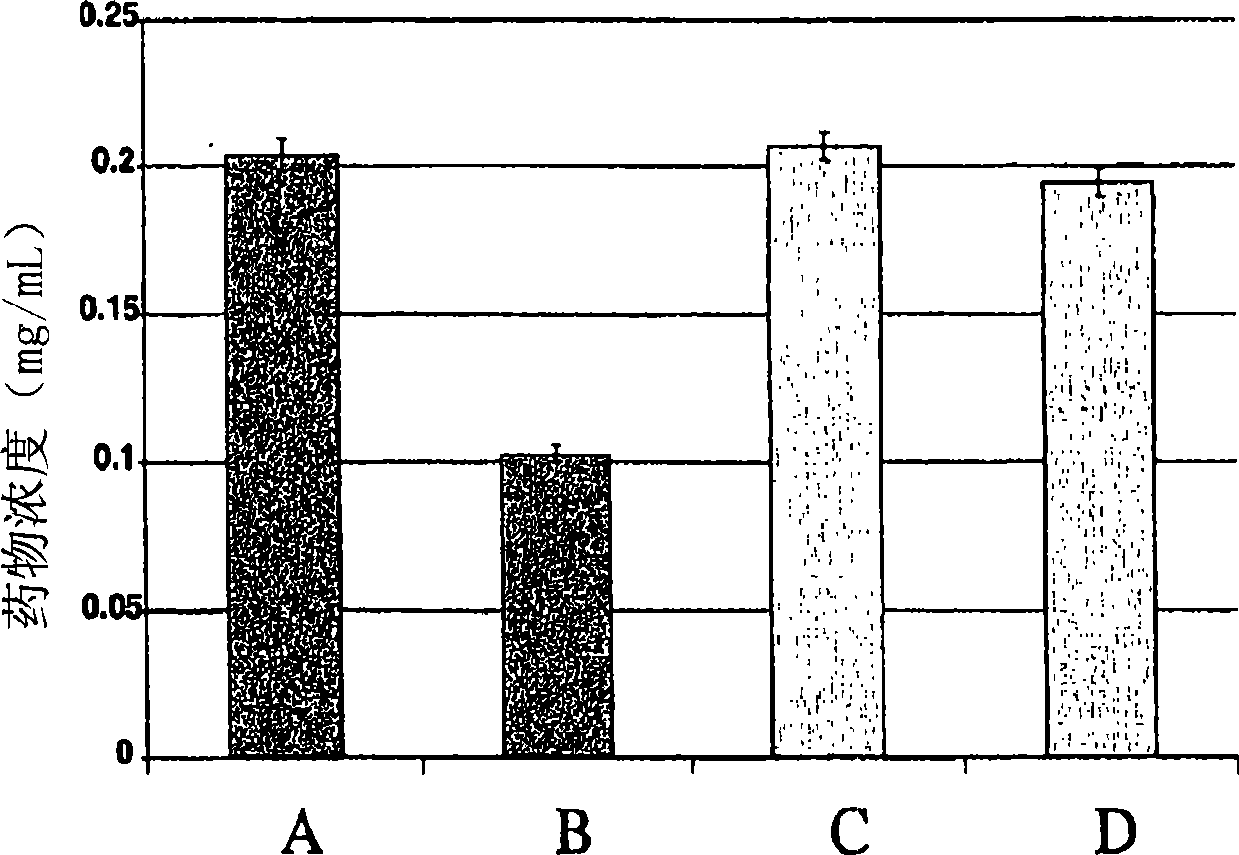
[0237] This example summarizes the solubility data and illustrates the composition of the formulations of the invention.
[0238] Solubility measurements of compounds were prepared into Wheaton 5 mL vials by adding 1 g of drug substance to 2 mL of oil. The headspace was blown with nitrogen and closed with a West 4405 / 50 gray Teflon coated plug and manually sealed with aluminum overseal. The preparation was repeated with oil containing purified egg or soy lecithin. Shake all labeled vials on a platform shaker for 18 hours at room temperature. After 18 hours, samples were diluted in mobile phase and analyzed by reverse-phase high-performance liquid chromatography (RP-HPLC). Table 2 summarizes the solubility results (expressed in mg / mL) of cinacalcet in different oil phases.
[0239] Table 2
[0240] Lecithin medium chain triglycerides Structure Triglycerides long chain triglycerides >341 >368 >340 Egg >349 >339 >362 soybean >327 >317 >...
example 2
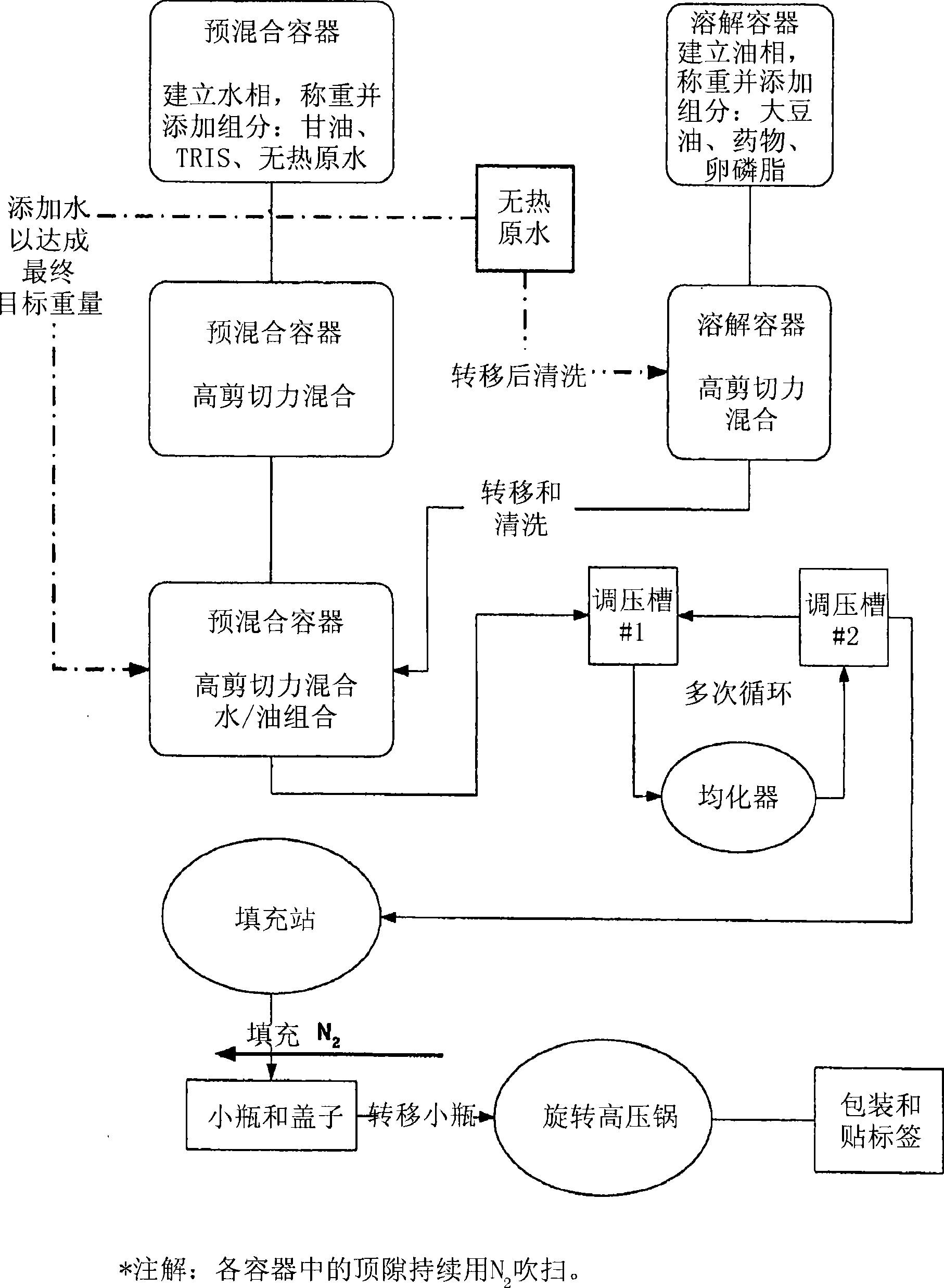
[0254] This example illustrates the different routes of preparation of the formulations of the invention.
[0255] Material:
[0256] Fresenius Kabi supplies purified egg lecithin, structured triglycerides (STG) and soybean oil or long chain triglycerides (LCT). Miglyol 810N or medium chain triglycerides (MCT) were purchased from Condea Chemie (formerly Huls). American Lecithin supplies soy lecithin. Other common laboratory chemicals (such as sodium hydroxide, hydrochloric acid, glycerin, isopropyl alcohol, TRIS, diethanolamine, etc.) were obtained from JT Baker, Sigma chemicals or other suitable suppliers business.
[0257] Coarse prototype emulsion formulations were prepared in batch sizes of 10 mL on an Ultraturrax T25 SI Janke and Kunkel paddle homogenizer equipped with a 10 mm hob stator, followed by a cell disrupting sonicator (Vibracell Ultrasonicator) with a titanium microtip probe. ) sonication. A batch size of 40 mL was prepared in a similar manner with the exce...
example 3
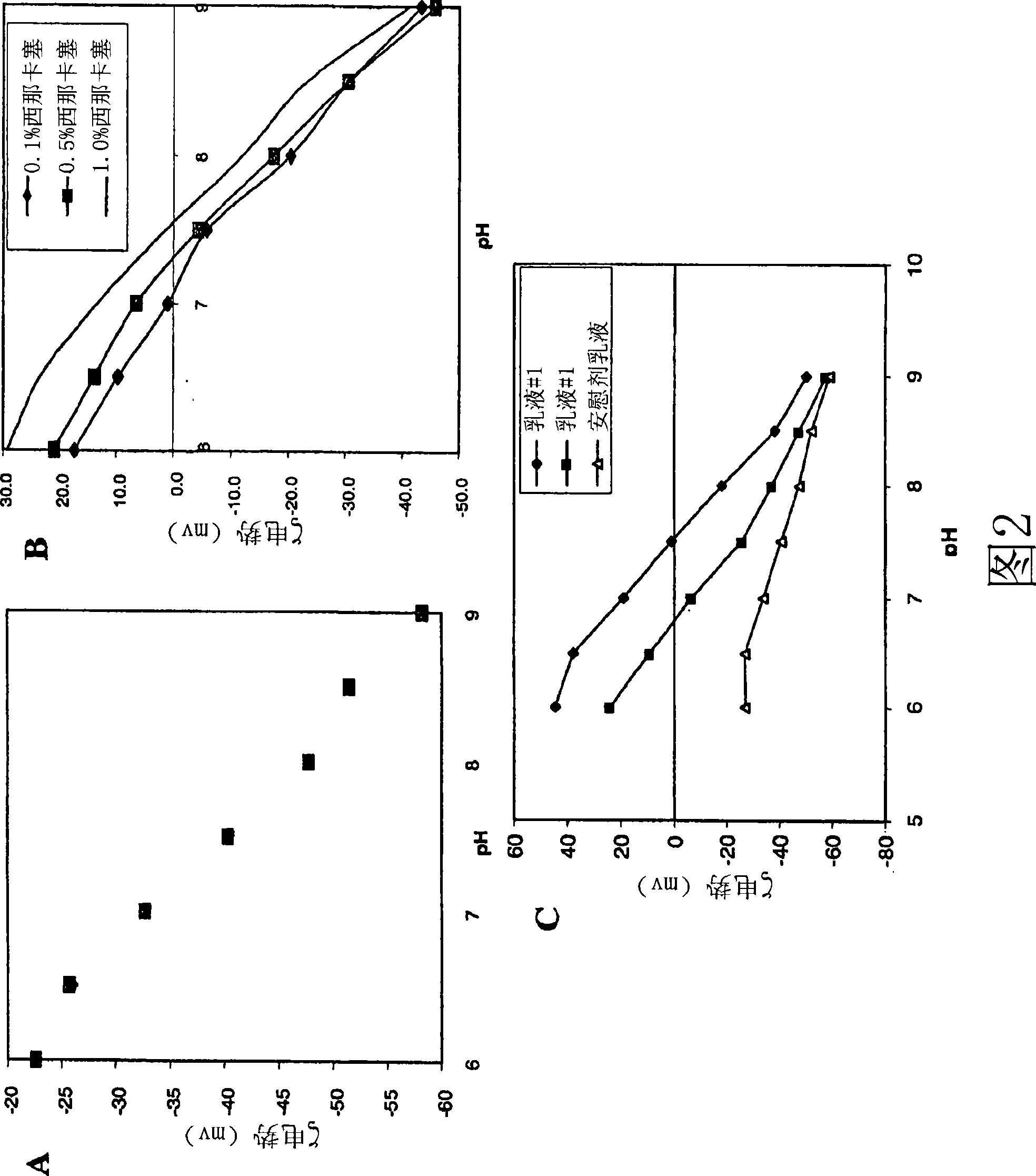
[0287] This example, as measured by the size and charge of the droplets, illustrates the effect of pH and buffer type on the stability of the formulations of the invention.
[0288] The results of these studies are depicted in Figure 2, which illustrate the effect of pH and the presence of calcimimetic compounds on droplet charge. Panel A: Titration results of 1% EYP / 10% SBO in 1 mM dibasic phosphate. Panel B: Titration results of cinacalcet 1% EYP / 10% SBO in 1 mM dibasic phosphate. The other components of the emulsion included 10% soybean oil, 1% egg lecithin, 2.25% glycerin and water. Panel C: Titration results for compound A. Emulsion composition: SBO 10%, lecithin 2%, pH7. The concentration of Compound A was 5 mg / mL for Formulation #1 and 1 mg / mL for Formulation #2. Emulsions with higher Compound A concentrations achieved neutral charge at a higher pH than emulsions with lower concentrations.
[0289] Zeta potential measurements were performed on a Malvern Zetasizer 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com