Immunoloregulation type DNA vaccine capable of preventing Eimeria necatrix
A DNA vaccine and Eimeria technology, applied in the field of DNA vaccines, can solve the problems of high cost, existence of scattered poison, difficult preservation, etc., and achieve the effects of long maintenance time, high safety and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of embodiment 1.NA4 gene
[0048] 1.1 Synthetic primers
[0049] According to the nucleotide sequence of the sporozoite surface antigen TA4 gene of Eimeria tenella, NA4 primers P1 and P2 were designed using the software primerpremier5.0, and sent to TaKaRa to synthesize primers. The sequence is as follows:
[0050] P1: 5'—G GGATCC GCTCGTCTCCTCT-3',
[0051] P2: 5'— GAATTCC TAGAAGAGAGCGAAA—3'.
[0052] The underlined parts are the introduced restriction sites BamHI and EcoRI, respectively; the boxed part is the initiation codon.
[0053] 1.2 Extraction of total RNA from sporozoites of poisonous Eimeria
[0054] A one-step method was used to extract the total RNA of sporulated oocysts, and the specific operation steps were as follows: Take about 10 sporulated oocysts of purified E.necatrix 7 Each was placed in a nuclease-free glass homogenizer treated with DEPC, and 1ml Trizol reagent was added, and rapidly ground for 5 minutes. Put the homogenized...
Embodiment 2
[0065] The preparation of embodiment 2.DNA vaccine
[0066] 2.1 Construction of recombinant plasmid pVAX-NA4 (see Figure 4 )
[0067] The cloned plasmid vectors pMD18-T-NA4 and pVAX1 were digested with BamHI and EcoRI respectively, and the NA4 target gene and pVAX1 large fragment were recovered, ligated according to an appropriate ratio, and the ligated product was transformed into competent Escherichia coli BL21, and the plasmid was extracted with BamHI and EcoRI It was identified as pVAX-NA4 by double enzyme digestion.
[0068] 2.2 Construction of recombinant plasmid pVAX-NA4-IL-2 (see Figure 5-8 )
[0069] 2.2.1 Synthetic primers
[0070] In order to construct the recombinant plasmid of tandem cytokines, use the software primer premier5.0 to design NA4 specific primer P3 without stop codon, and add coagulation factor X at the 3' end of NA4 a The specific hydrolysis sequence encodes the nucleotide sequence (box part), and the underlined part is the introduced enzyme cu...
Embodiment 3
[0075] The detection of embodiment 3.DNA vaccine expression situation in chicken body
[0076] The pVAX-NA4 and pVAX-NA4-IL-2 recombinant plasmids were extracted respectively, and 14-day-old chicks (100 μg / piece) were injected intramuscularly into the chest, and the injected site (breast muscle) and non-injected muscle (leg) were collected 7 days later.
[0077] 3.1 RT-PCR detection of DNA vaccine transcription
[0078] Total muscle RNA was extracted in one step, DNase I was added to remove residual recombinant plasmid and genomic DNA, and target genes were amplified by RT-PCR. The results showed that the DNA vaccine was transcribed in chicken muscle cells (see Figure 10 ).
[0079] 3.2 Detection of translation of DNA vaccine by Western blot
[0080] 3.2.1 Preparation of recombinant pET28a-NA4 protein antiserum
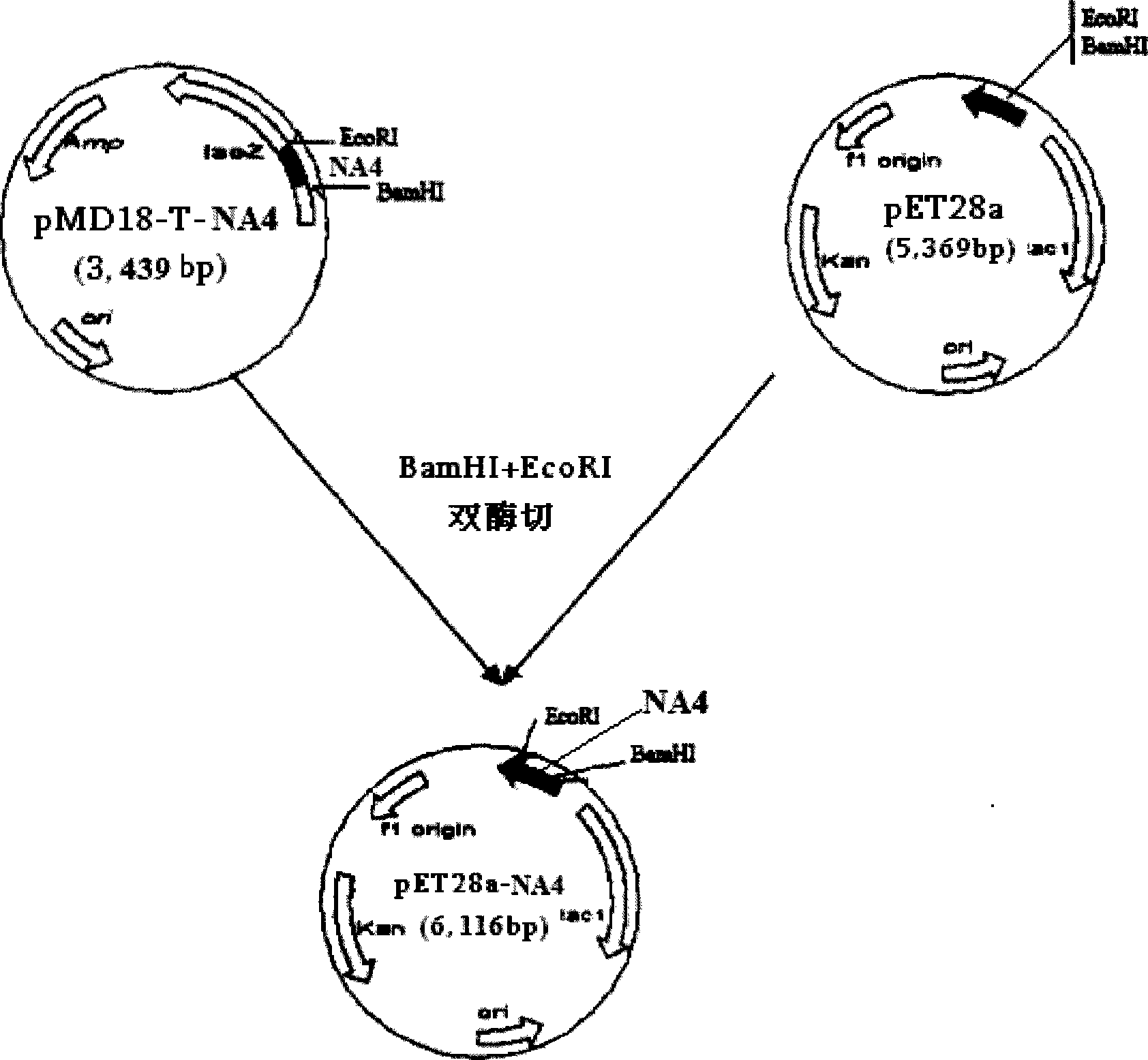
[0081] Transform pET28a-NA4 into Escherichia coli BL21 for prokaryotic expression. When the OD600 of the bacteria is 0.5, induce with isopropyl-β-D-thiogalactosi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com