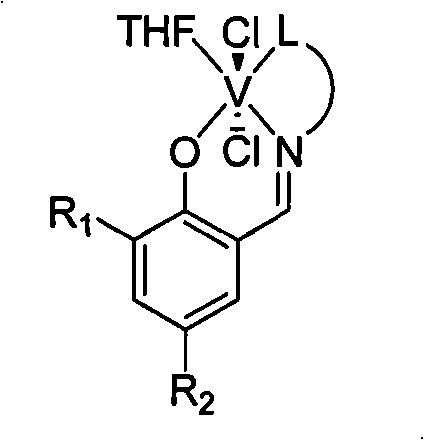
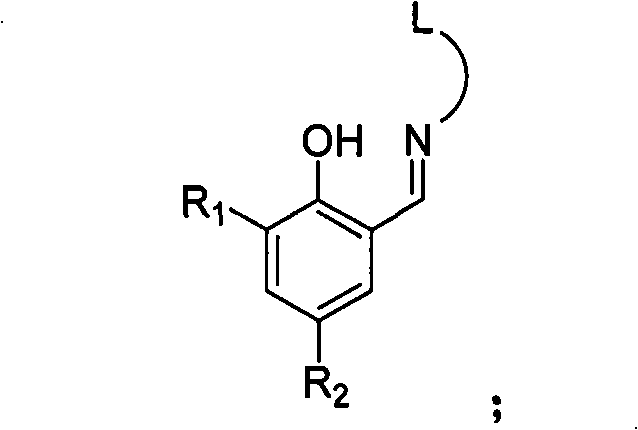
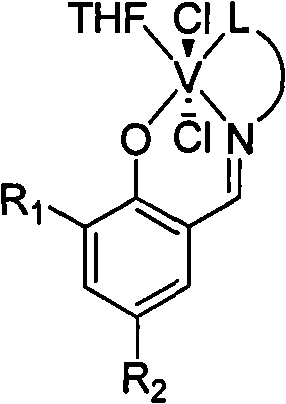
Three-tooth salicylaldehyde imine vanadium olefin polymerizing catalyst, preparation and uses thereof
A technology of tridentate salicylaldimine vanadium olefin and salicylaldimine vanadium olefin, which is applied in the field of tridentate salicylaldimine vanadium olefin polymerization catalyst and its preparation and application, and can solve the problems of poor high temperature resistance, weak copolymerization ability and low catalytic activity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]Add 9.77 g of salicylaldehyde equivalent to 80 mmol, 6.00 g of 2-methoxyethylamine equivalent to 80 mmol, 60 ml of methanol, and 2 ml of formic acid into a dry reactor, and react at 25° C. for 12 h. The solvent methanol was distilled off with a rotary evaporator, and 1000 ml of a solution of ethyl acetate and petroleum ether with a volume ratio of 1:100 was used as an eluting agent, and the residue was subjected to column chromatography to obtain 16.5 g of yellow solid Schiff's base. 92%. 1 HNMR (300MHz, CDCl 3 ): δ3.36(s, 3H, CH 3 ), 3.65(t, 2H, CH 2 N), 3.77(t, 2H, CH 2 O), 6.85-7.31 (m, 4H, Ar-H), 8.37 (s, 1H, CH=N), 13.41 (s, 1H, OH). According to mass spectrometry, the molecular ion peak m / e is 179. Elemental analysis measured value: C, 67.11%; H, 7.33%; N, 7.79%; theoretical value (C 10 h 13 NO 2 ): C, 67.02%; H, 7.31%; N, 7.82%.
[0027] Under a nitrogen atmosphere, add 0.27 g of the above-obtained Schiffer’s base equivalent to 1.5 mmol and 20 ml of anhyd...
Embodiment 2
[0034] 4.89g of salicylaldehyde is equivalent to 40mmol, 3.53g of N,N-dimethylethylenediamine is equivalent to 40mmol instead of 2-methoxyethylamine in Example 1, 30ml of methanol, 1ml of formic acid, and reacted at 25°C for 6h, The experimental operation was the same as in Example 1, and 7.15 g of yellow solid Schiff's base was obtained, with a yield of 93%. 1 H MR (300MHz, CDCl 3 ): δ2.30(s, 6H, CH 3 ), 2.63(t, 2H, CH 2 N), 3.73(t, 2H, CH 2 N), 6.84-7.32 (m, 4H, Ar-H), 8.37 (s, 1H, CH=N), 13.47 (s, 1H, OH). According to mass spectrometry, the molecular ion peak m / e is 192. Elemental analysis measured value: C, 68.70%; H, 8.40%; N, 14.58%; Theoretical value (C 11 h 16 N 2 O): C, 68.72%; H, 8.39%; N, 14.57%.
[0035] Under a nitrogen atmosphere, add 0.19 g of the above-obtained Schiffer’s base equivalent to 1.0 mmol and 10 ml of anhydrous tetrahydrofuran to a dry reactor, stir at room temperature for 10 min to dissolve the solid, add 26.4 mg of sodium hydride equivalen...
Embodiment 3
[0042] 7.33g of salicylaldehyde is equivalent to 60mmol, and 6.49g of 2-aminomethylpyridine is equivalent to 60mmol instead of 2-methoxyethylamine, 30ml of methanol, 1ml of formic acid in Example 1, and reacted at 25°C for 48h. The experimental operation is the same as the implementation Example 1, 10.19 g of yellow liquid Schiffer's base was obtained, and the yield was 80%.1 H NMR (300MHz, CDCl 3 ): δ4.95(s, 2H, CH 2 ), 6.77-7.72 (m, 7H, Ar-H and pyridine-H), 8.55 (s, 1H, CH=N), 8.48 (d, 1H, pyridine-H), 13.28 (s, 1H, OH). According to mass spectrometry, the molecular ion peak m / e is 212. Elemental analysis measured value: C, 73.69%; H, 5.72%; N, 13.24%; Theoretical value (C 13 h 12 N 2 O): C, 73.56%; H, 5.70%; N, 13.20%.
[0043] Under a nitrogen atmosphere, add 0.21 g of the above-obtained Schiffer’s base equivalent to 1.0 mmol and 10 ml of anhydrous tetrahydrofuran to a dry reactor, stir at room temperature for 10 minutes to dissolve the solid, add 36 mg of sodium hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com