Medical composition containing riluzole
A composition, the technology of riluzole, applied in the field of pharmaceutical composition, can solve the problems of low bioavailability, unstable stability, easy layering of emulsion, etc., and achieve the effect of improving dissolution rate, easy to carry, and convenient to take
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
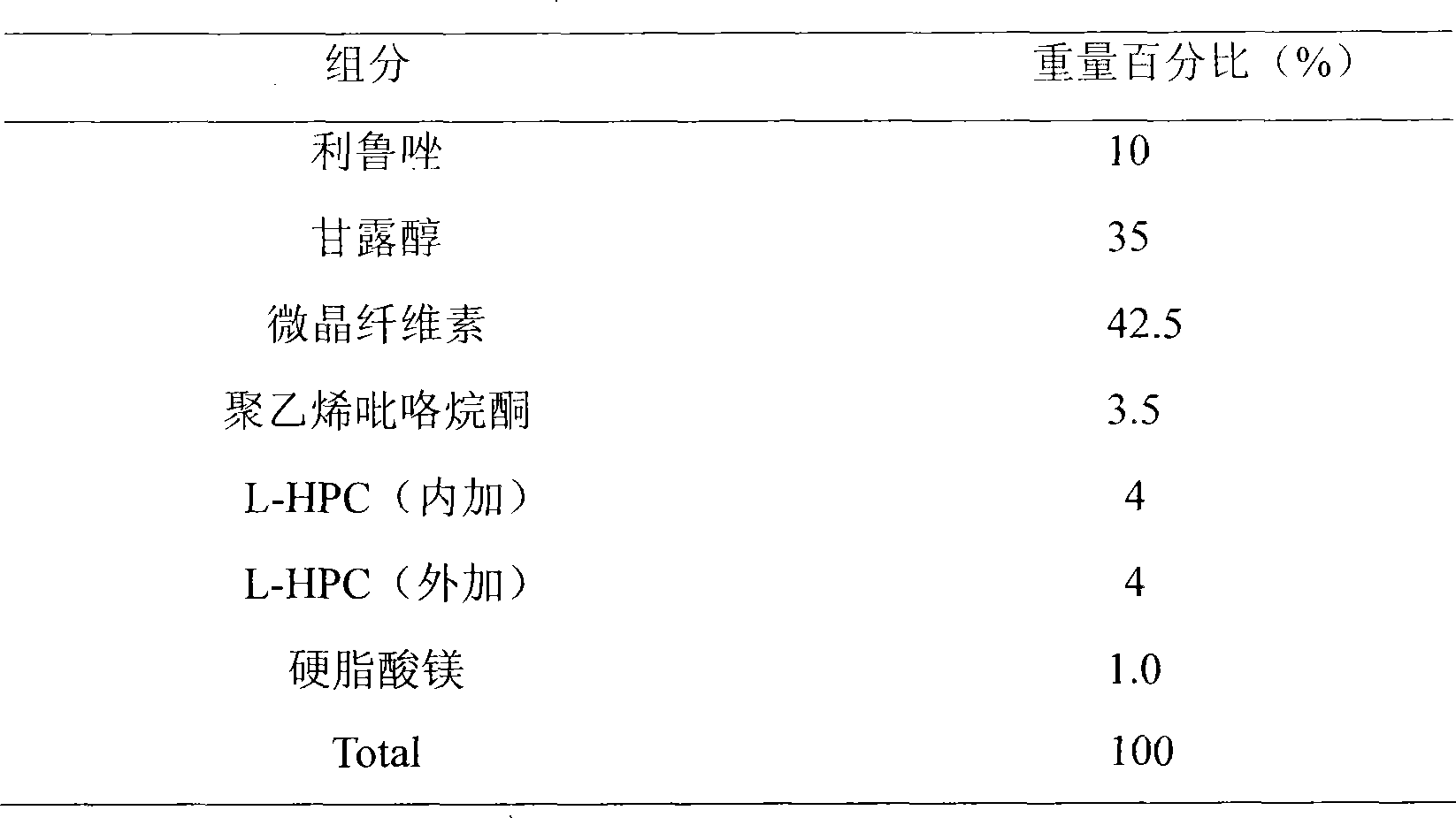
[0019]
[0020] Preparation:
[0021] Weigh the prescribed amount of riluzole, mannitol, microcrystalline cellulose, and L-HPC and mix evenly, weigh polyvinylpyrrolidone to make an aqueous solution as a binder for granulation, add the rest of L-HPC, and add stearic acid Magnesium, mixed, compressed into tablets, ready to use.
Embodiment 2
Total 100
[0024] Preparation:
[0025] Weigh the prescribed amount of riluzole, starch, sorbitol, and crospovidone and mix evenly; weigh hydroxypropyl methylcellulose to make an aqueous solution as a binder to granulate; add micropowder silica gel to mix evenly; pack into capsules Instantly.
Embodiment 3
Total 100
[0028] Preparation:
[0029] Weigh the prescribed amount of riluzole, lactose, microcrystalline cellulose, and partially cross-linked povidone and mix evenly, weigh hydroxypropyl methylcellulose to make an aqueous solution as a binder for granulation, and add the remaining part of cross-linked polyvinyl alcohol Vitamin ketone, adding magnesium stearate, mixing, tableting, to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com