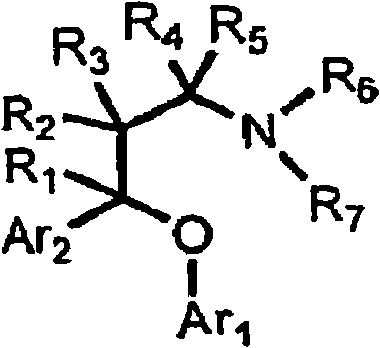
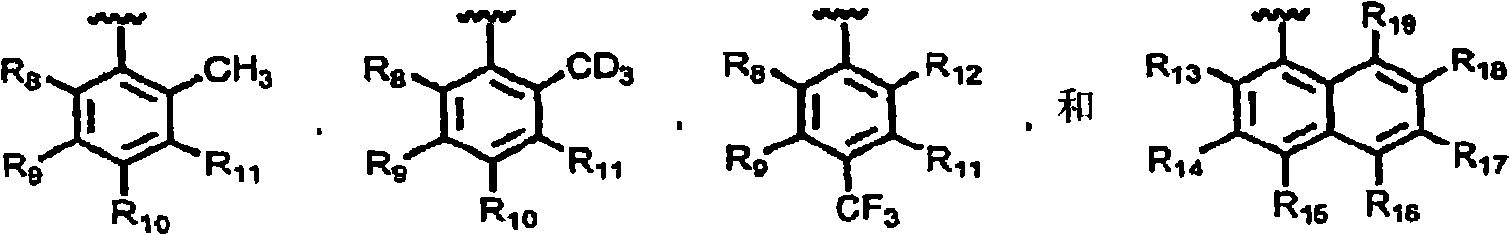
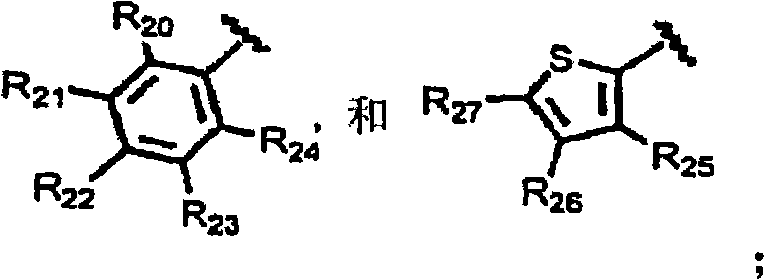
Substituted aryloxypropylamines with serotoninergic and/or norepinephrinergic activity
An isotope enrichment and compound technology, which can be used in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as increasing compound variability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0263] As used herein, and unless otherwise indicated, the following abbreviations have the following meanings: Me means methyl (CH 3 -), Et means ethyl (CH 3 CH 2 -), i-Pr refers to isopropyl ((CH 3 ) 2 CH 2 -), t-Bu refers to tert-butyl ((CH 3 ) 3 CH-), Ph refers to phenyl, Bn refers to benzyl (PhCH 2 -), Bz refers to benzoyl (PhCO-), MOM methoxymethyl, Ac refers to acetyl, TMS refers to trimethylsilyl, TBS refers to tert-butyldimethylsilyl, Ms methylsulfonyl (CH 3 SO 2 -), Ts refers to p-toluenesulfonyl (p-CH 3 PhSO 2 -), Tf refers to trifluoromethanesulfonyl (CF 3 SO 2 -), TfO refers to triflate (CF 3 SO 3 -), D 2 O refers to deuterium oxide, DMF refers to N,N-dimethylformamide, DCM refers to dichloromethane (CH 2 Cl 2 ), THF refers to tetrahydrofuran, EtOAc refers to ethyl acetate, Et 2 O refers to diethyl ether, MeCN refers to acetonitrile (CH 3 CN), NMP refers to 1-N-methyl-2-pyrrolidone, DMA refers to N, N-dimethylacetamide, DMSO refers to dimethylsu...
Embodiment 2-d3-3
[0274] Example 2-d 3 -3-Methyl-amino-1-thiophen-2-yl-propan-1-ol
[0275]
[0276]A solution of ethyl (3-hydroxy-3-thiophen-2-yl-propyl)-carbamate (0.367 g, 1.6 mmol) in dry tetrahydrofuran (5 mL) was dissolved at 0 °C under nitrogen atmosphere for 8 min Add dropwise to a stirred suspension of lithium aluminum deuteride (0.202 g, 4.81 mmol) in dry tetrahydrofuran (15 mL). The mixture was refluxed for 2 hours, cooled to 0 °C, treated dropwise with water (0.150 mL) for 10 minutes, stirred for 30 minutes, treated with ethyl acetate (50 mL), stirred for 30 minutes, treated with ethyl acetate (50 mL), room temperature Stir for 30 minutes and vacuum filter. The solid was rinsed with ethyl acetate (5 mL) and the filtrate was concentrated in vacuo to give 280 mg of a slightly cloudy off-white oil which solidified upon storage at -7°C overnight. The solid was taken up in ethyl acetate (5 mL), dried (Na 2 SO 4 ), filtered and concentrated in vacuo to give the desired product, d ...
Embodiment 3-d3
[0278] Example 3-d 3 -Methyl-[3-(naphthyl-1-yloxy)-3-thiophen-2-yl]-propyl]-amine (d 3 -B)
[0279]
[0280] Under nitrogen atmosphere, d 3 -3-Methyl-amino-1-thiophen-2-yl-propan-1-ol (0.133g, 0.760mmol) and sodium hydride (60% in mineral oil) (0.046g, 1.15mmol) in dimethyl ethylene Sulfone (2.0 mL) was stirred at 50°C for 40 minutes. 1-Fluoronaphthalene (0.167 mg, 1.15 mmol) was added and the mixture was stirred at 70 °C under nitrogen for 2 h, cooled to 0 °C, treated with water (5 mL, added over 1 min), stirred for 5 min and treated with acetic acid Ethyl ester (5x4mL) was extracted. The combined organic fractions were dried (Na 2 SO 4 ), filtered and concentrated in vacuo. The crude product was purified by column chromatography (dichloromethane-methanol-NH 4 OH) to give 60.8 mg of a clear light brown oil which was taken up in hexane (3 mL) and washed with concentrated aqueous sodium bicarbonate (2x1 mL), water (2x1 mL), concentrated aqueous sodium chloride (1 mL)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com