Methylphenidate hydrochloride sustained-release floating micropill in stomach and its preparation method
A technology of methylphenidate hydrochloride and sustained-release pellets, which is applied in the field of medicine, can solve problems such as poor safety, increased tensile strength, and smaller porosity, and achieve high safety in use, reduced side effects, and improved bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Ball core preparation:
[0024] Expandable polystyrene beads (particle size 180-300um) are softened when placed in a batch pre-expansion machine and heated by steam to above 92°C. Adjust the pre-expansion temperature, steam pressure and feed amount to volatilize the blowing agent The volume of the escaped particles slowly expands to 20 times, and then the foam particles are allowed to fall freely into the aging chamber made of mesh anti-static gauze for 8 hours. Enter the fluidized bed for drying to obtain pellet cores with a particle size of 500-800um. Then sieve according to the needs, and sieve out the pellet core of 700um-800um; its density is about 0.05g / cm 3 Then it is put into the fluidized bed and sprayed with stomach-dissolving coating liquid, which can further increase the particle diameter and density of the pellet core and make the appearance smoother.
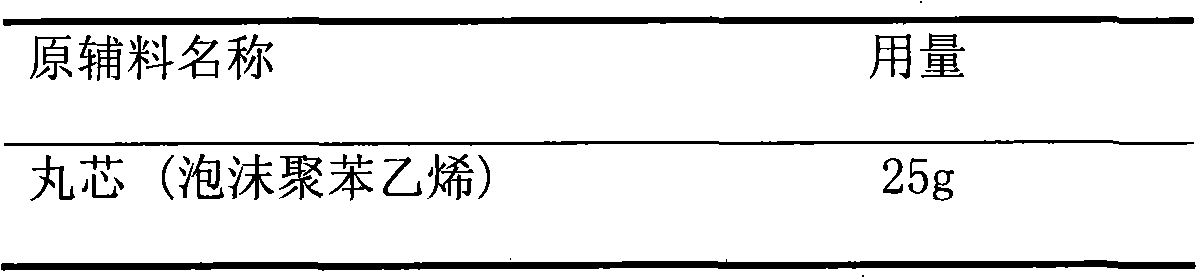
[0025] above core
[0026]
[0027] drug coating solution
[0028]
[0029] protective coating ...
Embodiment 2
[0046] Ball core preparation:
[0047] Expandable polystyrene beads (particle size 180-300um) are softened when placed in a batch pre-expansion machine and heated by steam to above 92°C. Adjust the pre-expansion temperature, steam pressure and feed amount to volatilize the blowing agent The volume of the escaped particles slowly expands to 20 times, and then the foam particles are allowed to fall freely into the aging chamber made of mesh anti-static gauze for 8 hours. Enter the fluidized bed for drying to obtain pellet cores with a particle size of 500-800um. Then sieve according to the needs, and sieve out the pellet core of 700um-800um; its density is about 0.05g / cm 3 Then it is put into the fluidized bed and sprayed with stomach-dissolving coating liquid, which can further increase the particle diameter and density of the pellet core and make the appearance smoother.
[0048] above core
[0049]
[0050] drug coating solution
[0051]
[0052] protective coating ...
Embodiment 3
[0069] Ball core preparation:
[0070] Expandable polystyrene beads (particle size 180-300um) are softened when placed in a batch pre-expansion machine and heated by steam to above 92°C. Adjust the pre-expansion temperature, steam pressure and feed amount to volatilize the blowing agent The volume of the escaped particles slowly expands to 20 times, and then the foam particles are allowed to fall freely into the aging chamber made of mesh anti-static gauze for 8 hours. Enter the fluidized bed for drying to obtain pellet cores with a particle size of 500-800um. Then sieve according to the needs, and sieve out the pellet core of 700um-800um; its density is about 0.05g / cm 3 Then it is put into the fluidized bed and sprayed with stomach-dissolving coating liquid, which can further increase the particle diameter and density of the pellet core and make the appearance smoother.
[0071] above core
[0072]
[0073] drug coating solution
[0074]
[0075] protective coating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com