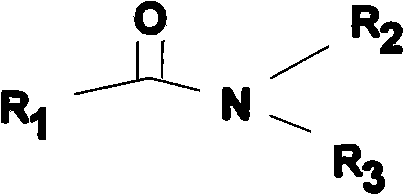
Esterification catalyst and method for synthesizing ester using the catalyst
A technology of esterification reaction and catalyst, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., to achieve the effect of reducing color, less reaction side reactions, and reducing corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
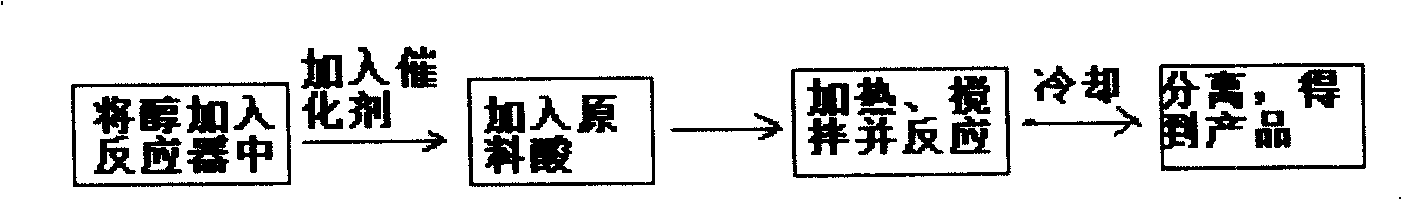
Image
Examples
Embodiment 1
[0030] The reaction between oleic acid and methanol is catalyzed by the catalyst prepared by mixing DMF and concentrated sulfuric acid.
[0031] A. After mixing DMF and concentrated sulfuric acid at a mol ratio of 1:1, take 0.95g of the mixture and add it to a four-necked flask filled with 48ml of methanol, then add 63ml of oleic acid, stir and reflux for 3h, the reflux temperature is 70 degrees Celsius, oil Acid conversion reached 97%. The acid value of the product is 5.9, and the color of the product methyl oleate is very light.
[0032] B, after mixing DMF and concentrated sulfuric acid at a mol ratio of 2:1, take 1.35g of the mixture and add it to a four-necked flask equipped with 48ml of methanol, then add 63ml of oleic acid, stir and reflux for 3h, the reflux temperature is 70 degrees Celsius, oil The acid conversion reached 96.3%. The acid value of the product is 7.3, and the color of the product methyl oleate is very light.
Embodiment 2
[0034] The reaction between oleic acid and methanol is catalyzed by a catalyst prepared by mixing urea and concentrated sulfuric acid.
[0035] After mixing urea and concentrated sulfuric acid at a molar ratio of 1:1.2, take 0.77g of the mixture and add it to a four-neck flask filled with 48ml of methanol, then add 63ml of oleic acid, stir and reflux for 3 hours, the reflux temperature is 70 degrees Celsius, the oleic acid The conversion rate reached 97.2%. , the acid value of the product is 5.6.
Embodiment 3
[0037] The corrosion of stainless steel in the reaction of oleic acid and methanol is catalyzed by a catalyst prepared by mixing DMF and sulfuric acid.
[0038] After mixing DMF and concentrated sulfuric acid at a mol ratio of 1:1, take 0.95g of the mixture and add it to a flask filled with 20ml of methanol, then add 63ml of oleic acid, and put a 4.5mm×1.8mm in size and a thickness of 1mm in it. 304 type stainless steel sheet, after reflowing at 80 degrees Celsius for 5 hours, the surface of the stainless steel sheet has no corrosion (corrosion degree <0.004%). When concentrated sulfuric acid is used as a catalyst under the same conditions, the corrosion degree of stainless steel sheet is 0.64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com