Preparation of TiO2/ZnFe2O4 magnetic composite photocatalyst
A technology of znfe2o4 and composite light, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., and can solve problems such as increased cost, complex synthesis process, and environmental hazards of organic matter , to achieve the effects of simplified production process, good dispersion, low reaction temperature and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Add zinc acetate and ferric nitrate at a molar ratio of 1:2 into an aqueous NaOH solution with a pH of 10, stir well to dissolve them, and react at 180°C for 24 hours to obtain ZnFe 2 o 4 precipitation.
[0021] (2) ZnFe obtained in step 1 2 o 4 Precipitate 2.41 g dispersed in 200 ml of V 水 :V 乙醇 =1:8 ethanol aqueous solution, ultrasonic for 30 minutes and then stirred for 2 hours, the pH of the solution was adjusted to 5 with glacial acetic acid to obtain solution A.
[0022] (3) Add 3.4 ml of tetrabutyl titanate into 20 ml of ethanol, and stir for 30 minutes to obtain solution B.
[0023] (4) All the solution B obtained in step 3 was dropped into the solution A obtained in step 2 dropwise, and after stirring for 10 hours, the red precipitate C was obtained by centrifugal separation and deionized water washing.
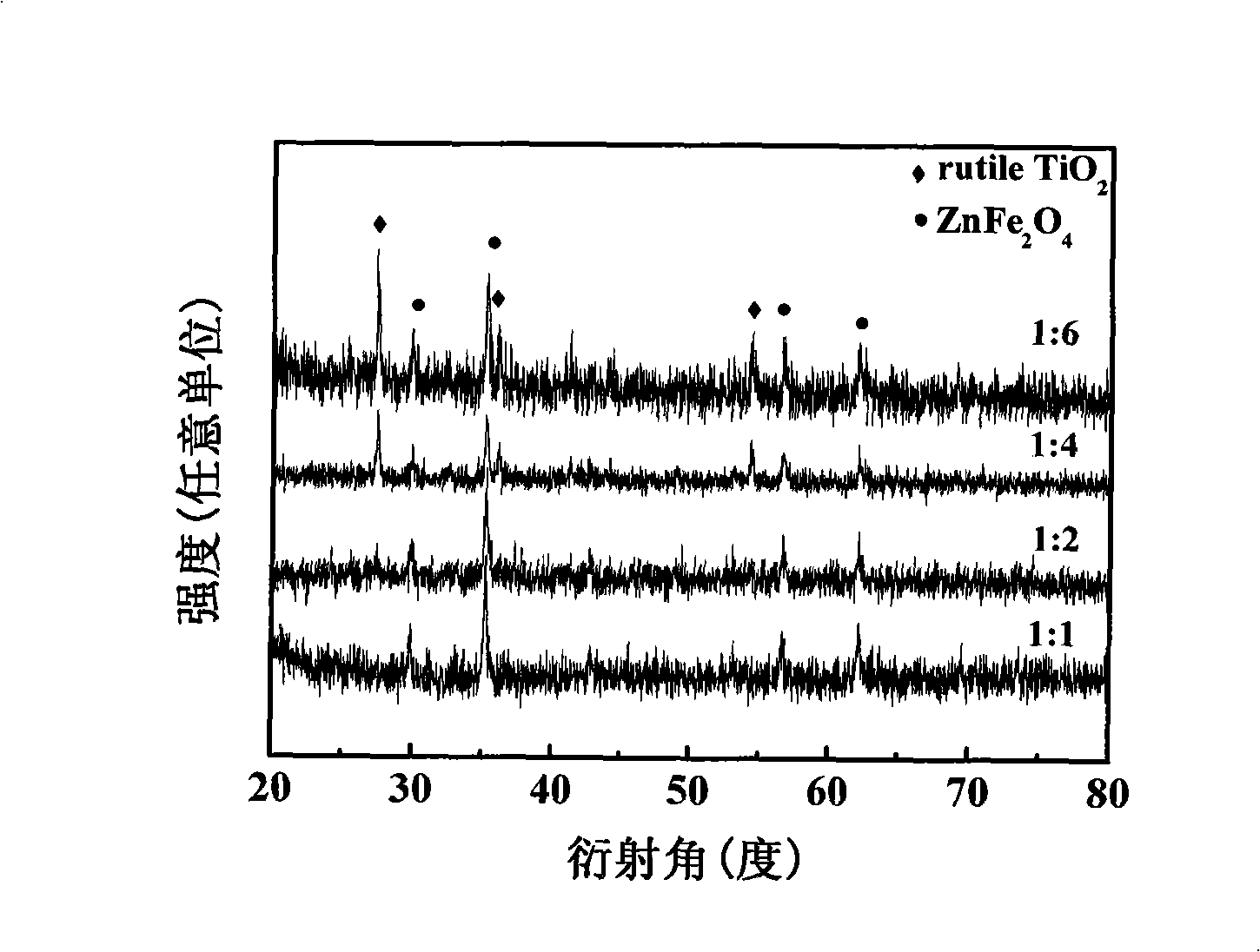
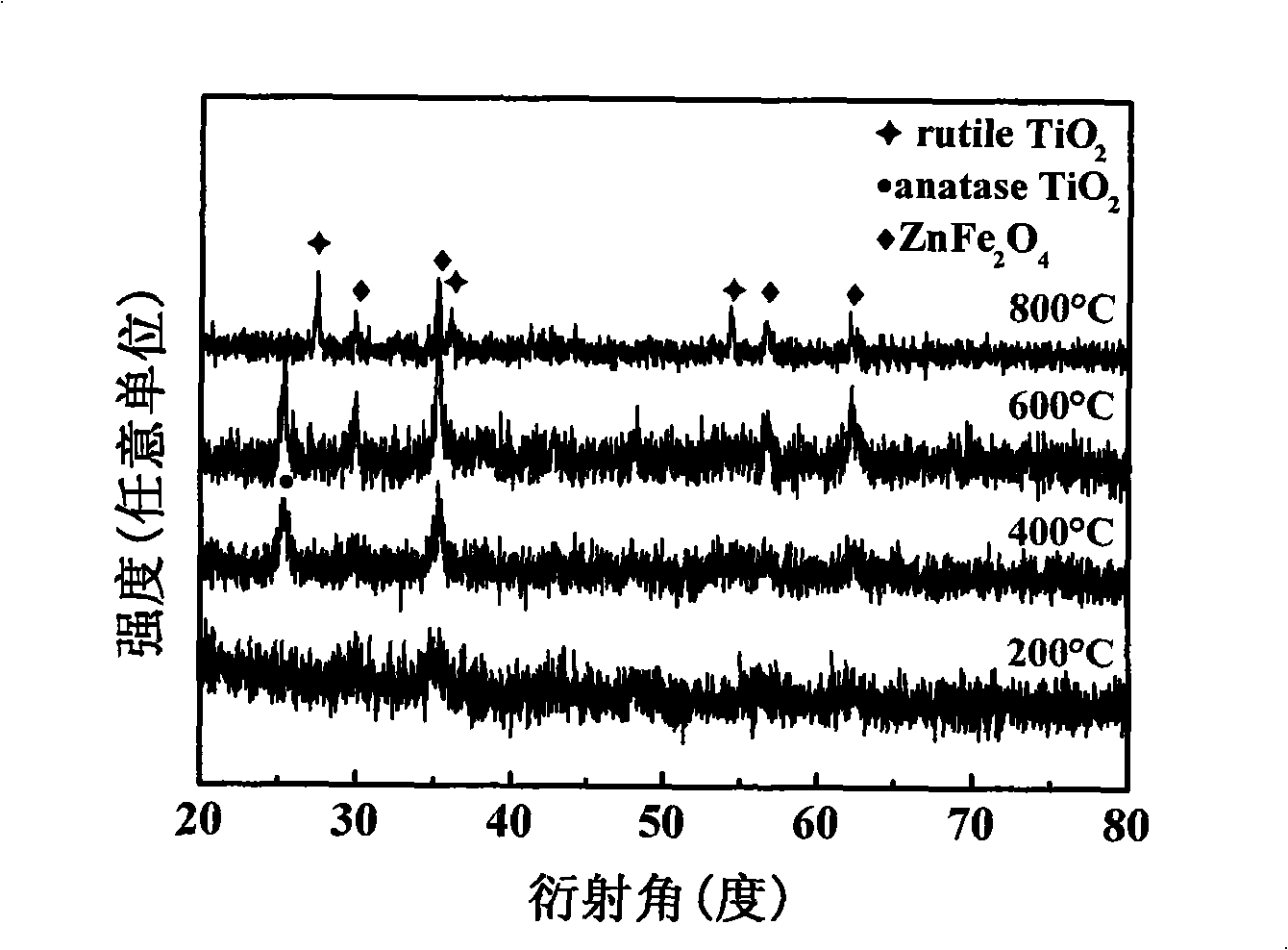
[0024] (5) Calcining the red precipitate C obtained in step 4 at 800° C. for 4 hours to obtain the desired product. The powder X-ray diffraction pa...
Embodiment 2
[0026] (1) Add zinc acetate and ferric nitrate at a molar ratio of 1:2 into an aqueous NaOH solution with a pH of 10, stir well to dissolve them, and react at 180°C for 24 hours to obtain ZnFe 2 o 4 precipitation.
[0027] (2) ZnFe obtained in step 1 2 o 4 Precipitate 2.41 g dispersed in 200 ml of V 水 :V 乙醇 =1:8 ethanol aqueous solution, ultrasonic for 30 minutes and then stirred for 2 hours, the pH of the solution was adjusted to 5 with glacial acetic acid to obtain solution A.
[0028] (3) Add 6.8 ml of tetrabutyl titanate into 20 ml of ethanol, and stir for 30 minutes to obtain solution B.
[0029] (4) All the solution B obtained in step 3 was dropped into the solution A obtained in step 2 dropwise, and after stirring for 10 hours, the red precipitate C was obtained by centrifugal separation and deionized water washing.
[0030] (5) Calcining the red precipitate C obtained in step 4 at 800° C. for 4 hours to obtain the desired product.
[0031] Such as figure 1 , ...
Embodiment 3
[0033](1) Add zinc acetate and ferric nitrate at a molar ratio of 1:2 into an aqueous NaOH solution with a pH of 10, stir well to dissolve them, and react at 180°C for 24 hours to obtain ZnFe 2 o 4 precipitation.
[0034] (2) ZnFe obtained in step 1 2 o 4 Precipitate 2.41 g dispersed in 200 ml of V 水 :V 乙醇 =1:8 ethanol aqueous solution, ultrasonic for 30 minutes and then stirred for 2 hours, the pH of the solution was adjusted to 5 with glacial acetic acid to obtain solution A.
[0035] (3) Add 12.6 ml of tetrabutyl titanate into 20 ml of ethanol, and stir for 30 minutes to obtain solution B.
[0036] (4) All the solution B obtained in step 3 was dropped into the solution A obtained in step 2 dropwise, and after stirring for 10 hours, the red precipitate C was obtained by centrifugal separation and deionized water washing.
[0037] (5) Calcining the red precipitate C obtained in step 4 at 800° C. for 4 hours to obtain the desired product.
[0038] Such as figure 1 , 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com