Application of 2',4'-dihydroxy-6'-methoxy-3',5'-dimethyl chalcone as PPAR gamma agonist
A technology of dimethylchalcone and gamma agonist, applied in the direction of ketone active ingredients, metabolic diseases, drug combination, etc., can solve the problems of increasing congestive heart failure, edema, weight gain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
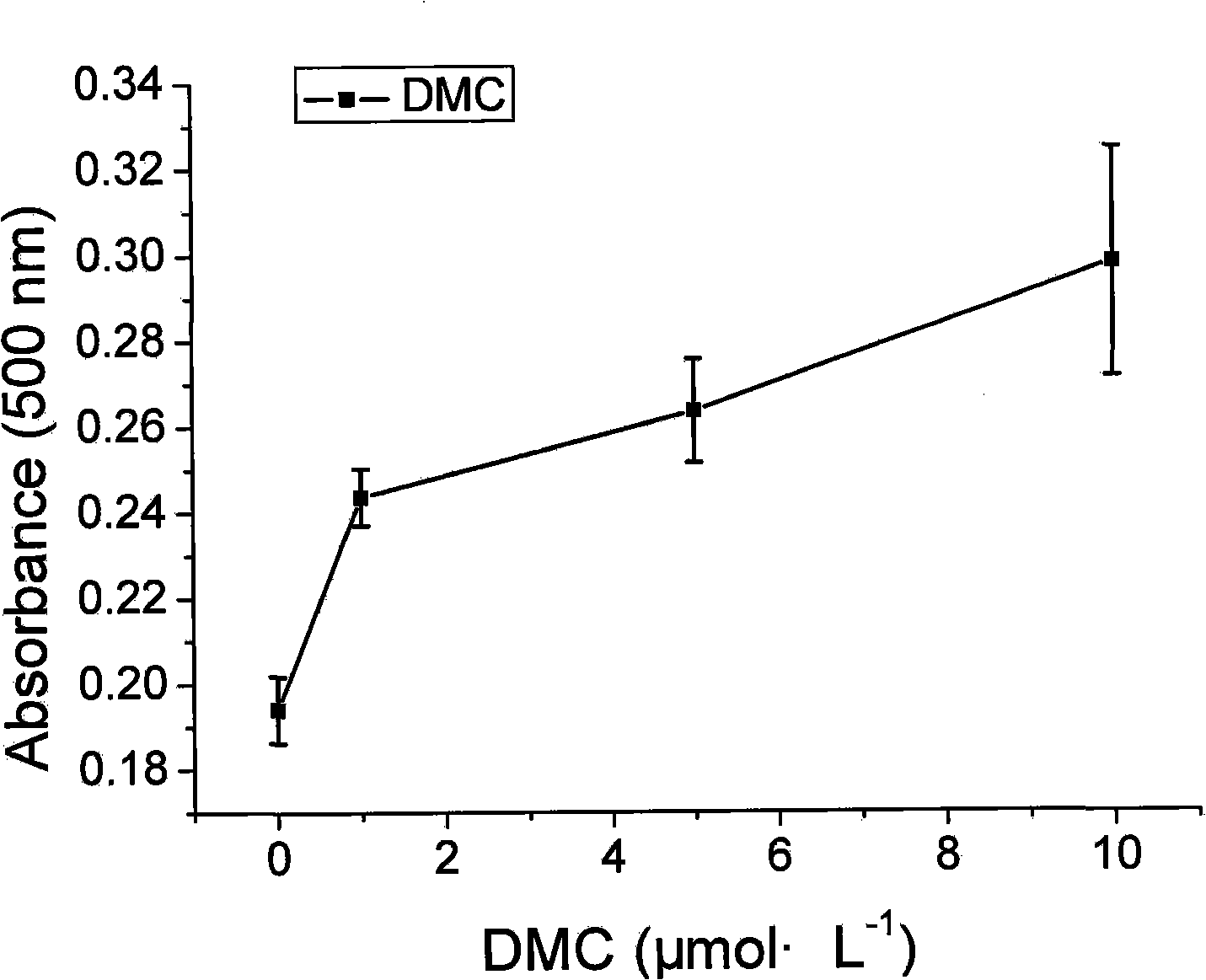
[0016] Example 1 Analysis of PPARγ ligand-binding activity of DMC
[0017] 293T cells in the logarithmic growth phase were added to each well at 1.5×10 4 Cells were inoculated in 96-well culture plates, cultured overnight, and used for transfection when the cells grew to 80%-90% full. Dilute Lipofectamine2000 (volume ratio: 0.5:100) and plasmid DNA with OPTI-MEM medium, mix the diluted liposomes and plasmid DNA in equal volumes, place at room temperature for 20 minutes, and then transiently co-transfect the cells. The amount of plasmid DNA used was: GAL4-PPARγ-LBD 25ng per well, pFR-Luc 50ng per well, pRL-TK-Renilla 5ng per well. Incubate for 4 hours after transfection before adding drugs, the concentration of the experimental group is from 1n mol L -1 to 10μmol·L -1 The same volume of DMSO was added to the normal control group, and the luciferase activity was detected 24 hours after the addition of the drug. The detection method was strictly in accordance with the instruct...
Embodiment 2D
[0019] Example 2 Effect of DMC on the differentiation of 3T3-L1 preadipocytes
[0020] The 3T3-L1 preadipocytes cultured in a 24-well plate were grown to complete confluence 2 days later, and then replaced with induction solution (containing 200nmol·L -1 Insulin, 1 μmol L -1 Dexamethasone was cultured in 10% FBS high glucose DMEM), and at the same time, each well was added with a final concentration of 1 μmol L -1 , 5μmol·L -1 and 10 μmol L -1 DMC, the wells added with DMSO were used as normal controls, and after 3 days of induction, they were replaced with 200nmol·L -1 Insulin in 10% FBS High Glucose DMEM. After 2 days, the incubation was finished, and oil red O staining was performed: absorb all the medium, wash the cells once with PBS, fix the cells with 10% formaldehyde for 24 hours, discard the fixative, wash once with 60% isopropanol, and wait for the cells to fully After drying, add Oil Red O solution, stain at room temperature for 30 min, discard the dye, and wash...
Embodiment 3
[0022] Example 3 Effect of DMC on glucose uptake in 3T3-L1 adipocytes
[0023] The 3T3-L1 adipocytes in the 24-well culture plate were treated with 5 μmol·L -1 DMC and 5 μmol L -1 Rosiglitazone treated for 48h, DMSO treated cells as a normal control group. Before the glucose uptake test, the cells were replaced with 0.1% FBS-containing DMEM medium for starvation culture for 2 h, the medium was aspirated, the cells were washed with PBS three times, and replaced with 2% BSA-containing KRH buffer (20 mM HEPES, pH 7.4 ;136mmol L -1 NaCl; 4.7mmol L -1 KCl; 1.25mmol L -1 MgSO 4 ; 1.25mmol L -1 CaCl 2 ) at 37°C for 30 min, and the final concentration was 200 nmol L -1 Insulin continued to incubate at 37°C for 30min, then add 20μl containing 1.6μCi to each well [ 3 H]-2-deoxyglucose and 2-deoxyglucose mixed solution, incubate at 37°C for 10 minutes, quickly absorb the incubation solution, wash the cells 3 times with ice-cold PBS, and blow and lyse the cells with scintillat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com