Novel cellulase and uses thereof
A cellulase, cellulose technology, applied in the application, enzyme, enzyme and other directions, can solve problems such as inability to adapt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
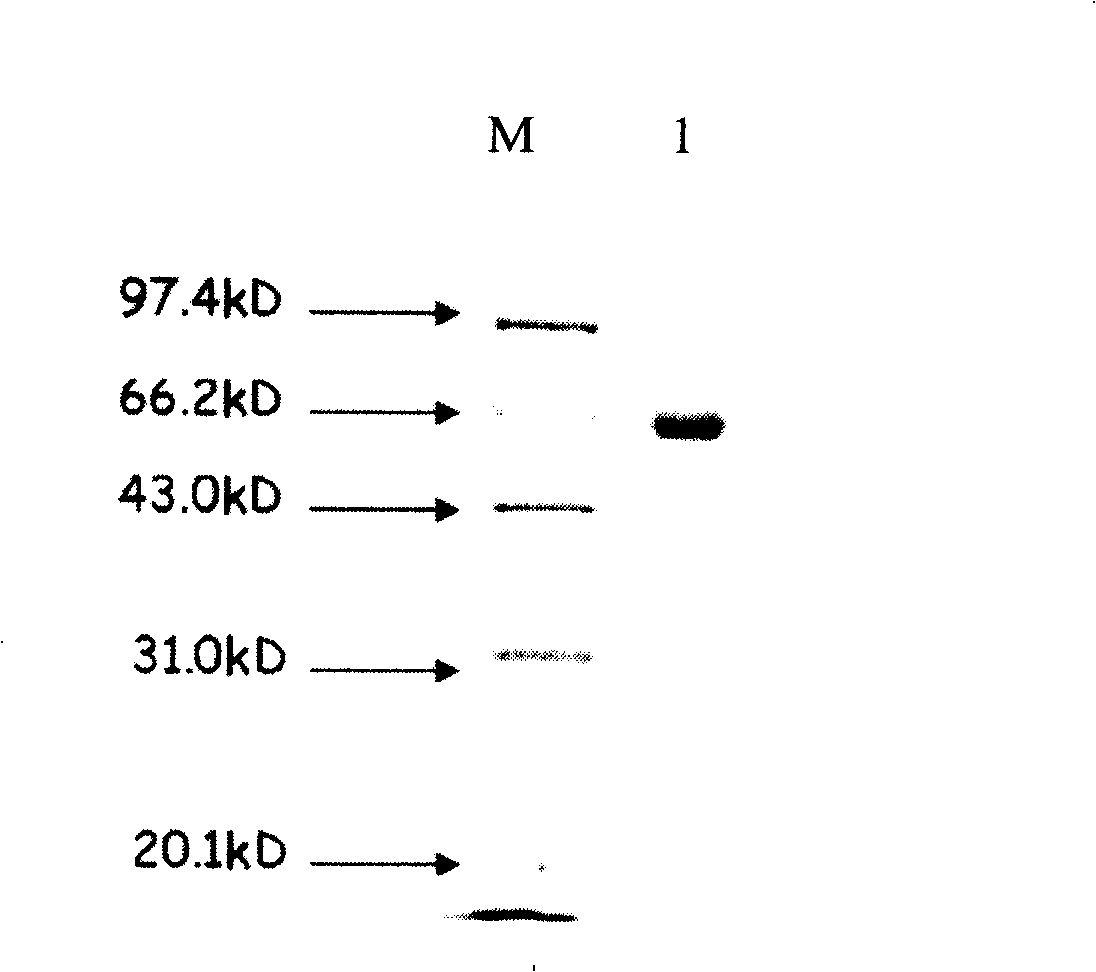
[0089] Example 1: Separation and purification of apple snail EG65
[0090] 1. Materials and Reagents
[0091] Apple snails are widely distributed in Fujian, Guangdong, Guangxi, Zhejiang, Jiangsu and other provinces in China. Apple snails purchased from Xiamen, Fujian were used as experimental materials. In the experiment, the chromatography media DEAE-Sepharose CL-6B, DEAE-Sepharose fastflow DEAE-Sephadex A-50, Phenyl-Sepharose CL-4B were purchased from Pharmacia AB (Sweden), and Bio-gel P-100 was purchased from Bio-Rad (USA) Company. The electrophoresis reagents acrylamide, methylene bisacrylamide and sodium dodecylsulfonate (SDS) were purchased from Pharmacia AB (Sweden), and protein molecular weight standards were purchased from Shanghai Sebastian Company. Bioassay reagents pNPC, Sigmacell 101, xylan (xylan from birchwood or oat spellt), CMC-Na (medium viscosity), starch (from potato) were purchased from Sigma Company; β-salicin, Avicel pH101 were purchased from Fluka Co...
Embodiment 2
[0107] Example 2: Activity assay of EG65
[0108] 1. Activity assay method
[0109] (1) Glycoprotein identification
[0110] Fluorescent hydrazide (DNS-Gly-NHNH2) was used to specifically label sugar chains on glycoproteins, according to W.Y.Liu, R.Q.Jiang in "The fluorescent labeling of glycoproteins on sodium dodecyl sulfate-polyacrylamide gel" (Bioorg.Chem.22(1994) 29-35) to identify glycoproteins.
[0111] (2) Determination of optimum pH, optimum temperature and pH stability and temperature stability
[0112] 1) Optimum pH: Take an appropriate amount of EG65 and add it to 200μl of different pH values, dissolve it in 100mMC at pH3.6-7.2 6 h 8 o 7 (citric acid) / Na 2 HPO 4 2H 2 The concentration of O was 1% (w / v) in CMC-Na, and the activity was measured according to the standard method.
[0113] 2) Optimum temperature: measure the activity according to the standard method within the range of 25°C to 70°C.
[0114] 3) pH stability: the EG65 enzyme was incubated at 50...
Embodiment 3
[0133] Embodiment 3: Cloning and expression of the gene of EG65 and its related genes
[0134] 1. Materials, Reagents and Methods
[0135] (1) Materials and reagents
[0136] 3-cyclohexylamino-1-propanesulfonic acid (CAPS) transfer buffer was purchased from Amresco; V8 proteolytic enzyme was purchased from Pierce; dithiothreitol (DTT) was purchased from Sigma; PVDF membrane was purchased from Millipore company; Trizol was purchased from Shanghai Sangong (Canada BioBasic Inc. imported packaging); reverse transcription reagents were purchased from Promega; , Pyrobest E, La-Taq E, etc.) and the carrier pMD18-T Vector were purchased from Takara; the gel recovery kit and Ponceau were purchased from Shanghai Huashun Bioengineering Co., Ltd.; the plasmid extraction kit was purchased from Broadtech; IPTG Purchased from BBI Company; X-gal was purchased from Takara Company; the peptone (Tryptone) and yeast extract (Yeast Extract) used in the cultivation of E.coli were purchased from O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com