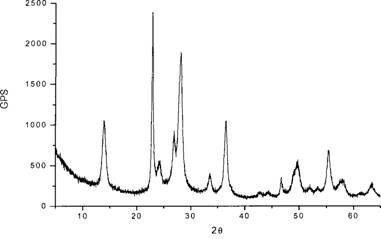
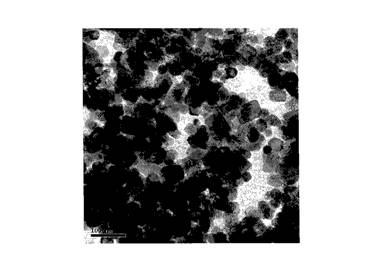
Method for synthesizing sensitive photochromic WO3 nano powder by formaldehyde inducement
A photochromic and nano-powder technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, tungsten oxide/tungsten hydroxide, etc., can solve the problems of complex preparation methods, weak photochromic ability, and less powder research , to achieve the effect of simple synthesis method, enhanced surface activity and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A formaldehyde-induced synthesis of sensitive photochromic WO 3 The method for nanometer powder, it comprises the steps:
[0018] First, potassium tungstate was used as the tungsten source (K 2 WO 4 ), to prepare 1mol / L of K 2 WO 4 Solution 100ml, and under the effect of 500r / min stirring speed of magnetic stirrer, dropwise add hydrochloric acid, the pH value of adjustment solution is 0.8, under the action of magnetic stirring, then add dropwise formaldehyde solution 8ml as inducer, the volume of formaldehyde solution The concentration is 38%, stirred and homogenized for 4 hours; then the obtained reaction system is put into a hydrothermal kettle for 60 hours of hydrothermal reaction at 120° C.; the reaction product is first washed repeatedly with distilled water (double distilled water) until the pH of the clear liquid is 7.0, then wash with absolute ethanol to remove residual water-soluble impurities and excess formaldehyde; finally, put the reacted powder into a ...
Embodiment 2
[0028] The difference from Example 1 is that the amount of formaldehyde solution added is 0.8 times that of Example 1, and the measured chromaticity value of the powder is 2.953. After 20 minutes of ultraviolet light irradiation, the powder turns blue, and its chromaticity value is 35.874 , Moved into a dark place for 1.5h and faded, its chromaticity value was 3.015, and its powder particle size was 35.6nm.
Embodiment 3
[0030] The difference from Example 1 is that the same sample was tested for its photochromic performance between 12:00 noon and 14:00 on a sunny summer day in southern China. After 20 minutes of light, the chromaticity value was 42.362, and it faded after being moved into a dark place for 2 hours. The degree value is 3.042.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com