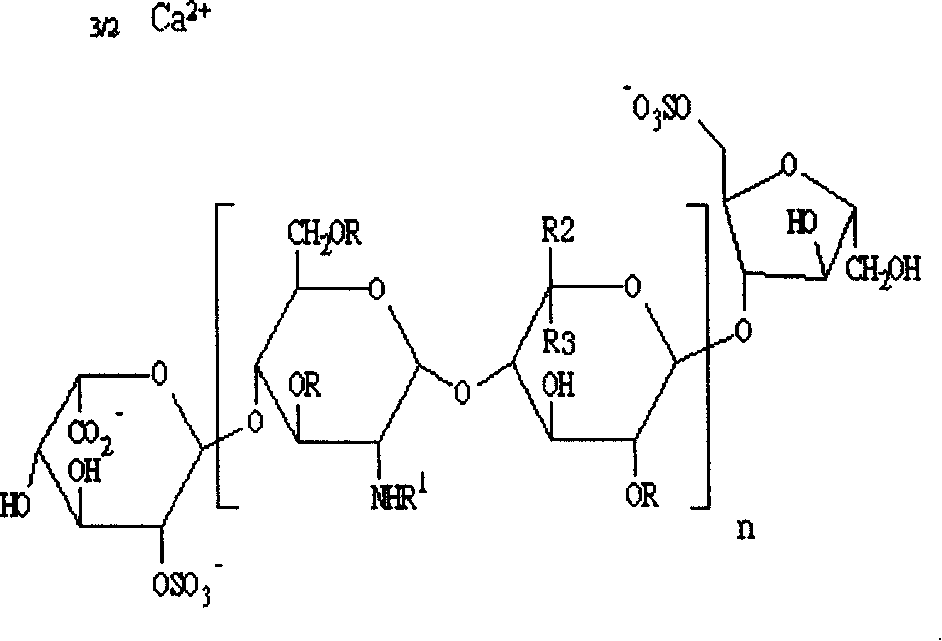
Low molecule heparin sustained-release controlled-release capsule preparations and preparation method thereof
A low-molecular-weight heparin and capsule technology, used in pharmaceutical formulations, blood diseases, extracellular fluid diseases, etc., can solve the problems of inconvenient administration, pain and possible infection at the injection site, reducing the number of administrations, affirming the curative effect, Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] The weight composition of low-molecular-weight heparin pellet core includes (dosage per 1000 sustained-release capsules)
[0037] Lactose or fructose or sucrose 40g
[0038] Starch or microcrystalline cellulose 40g
[0039] Hydroxypropyl methylcellulose or macrogol or tragacanth 2g
[0040] The weight composition of the drug-containing layer of low-molecular-weight heparin pellets includes (consumption per 1000 sustained-release capsules)
[0041] Low molecular weight heparin 40g
[0042] Lactose or fructose or sucrose 40g
[0043] Starch or microcrystalline cellulose 40g
[0044] Hydroxypropyl methylcellulose or macrogol or tragacanth 2g
[0045] The weight composition of coating layer of low-molecular-weight heparin pellets includes (per 1000 sustained-release capsules dosage)
[0046] Polyacrylic resin or ethyl cellulose 30g
[0047] Plasticizer: castor oil or diethyl phthalate 5g
[0048] 95% ethanol 1000g
[0049] Static remover talcum powder 10g.
example 2
[0051] The weight composition of low-molecular-weight heparin pellet core includes (dosage per 1000 sustained-release capsules)
[0052] Lactose or fructose or sucrose 160g
[0053] Starch or microcrystalline cellulose 160g
[0054] Hydroxypropyl methylcellulose or polyethylene glycol or tragacanth gum 10g
[0055] The weight composition of the drug-containing layer of low-molecular-weight heparin pellets includes (consumption per 1000 sustained-release capsules)
[0056] Low molecular weight heparin 160g
[0057] Lactose or fructose or sucrose 160g
[0058] Starch or microcrystalline cellulose 160g
[0059] Hydroxypropyl methylcellulose or polyethylene glycol or tragacanth gum 10g
[0060] The weight composition of coating layer of low-molecular-weight heparin pellets includes (per 1000 sustained-release capsules dosage)
[0061] Polyacrylic resin or ethyl cellulose 100g
[0062] Plasticizer: castor oil or diethyl phthalate 15g
[0063] 95% ethanol 1500g
[0064] Antis...
example 3
[0066] The weight composition of low-molecular-weight heparin pellet core includes (dosage per 1000 sustained-release capsules)
[0067] Lactose or fructose or sucrose 100g
[0068] Starch or microcrystalline cellulose 100g
[0069] Hypromellose or macrogol or tragacanth 6g
[0070] The weight composition of the drug-containing layer of low-molecular-weight heparin pellets includes (consumption per 1000 sustained-release capsules)
[0071] Low molecular weight heparin 100g
[0072] Lactose or fructose or sucrose 100g
[0073] Starch or microcrystalline cellulose 100g
[0074] Hypromellose or macrogol or tragacanth 6g
[0075] The weight composition of coating layer of low-molecular-weight heparin pellets includes (per 1000 sustained-release capsules dosage)
[0076] Polyacrylic resin or ethyl cellulose 80g
[0077] Plasticizer: castor oil or diethyl phthalate 10g
[0078] 95% ethanol 1200g
[0079] Static remover talcum powder 20g.
[0080] The preparation method of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com