Method for preparing human chorion gonadotrophic hormone beta subunit
A chorionic gonadotropin and beta subunit technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problem of low cost, excessive glycosylation, and reduction of renaturation rate to zero and other problems to achieve the effect of solving source shortage and efficient and correct expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
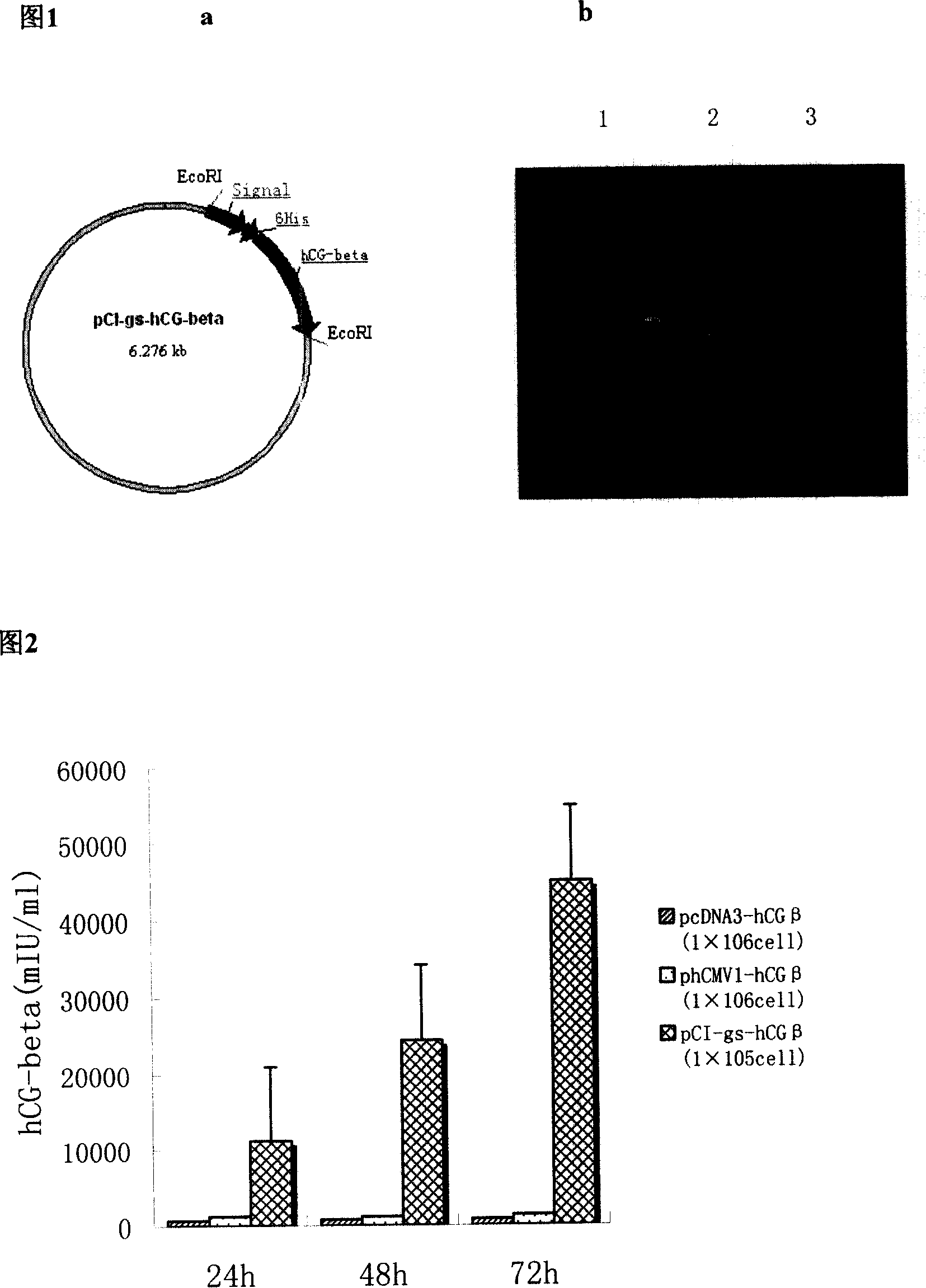
[0025] 1) Construction of secreted eukaryotic expression vector pCI-gs-signal-6His-hCG β
[0026] Using the plasmid phCMV1-signal-6His-hCG β as a template, two pairs of PCR primers were designed to amplify hCG β cDNA by PCR technology, with EcoR I, secretion signal peptide and 6His at the 5-end, and a stop codon at the 3-end , EcoR I restriction sites, PCR product gel recovery, and those with correct sequencing results were respectively connected to the pMD18-T vector for enzyme digestion identification and sequencing.
[0027] Digest pMD18-T-signal-6His-hCG β and pCI-gs with EcoR I respectively, recover the target gene fragment and the pCI-gs carrier part from the gel, transform the product of the ligation reaction into Top 10F'competent, cultivate and perform rapid screening, and select A small amount of positive colonies were extracted and identified by plasmid digestion. Take the cloned strains with correct enzyme digestion results and inoculate them, extract a large numb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com