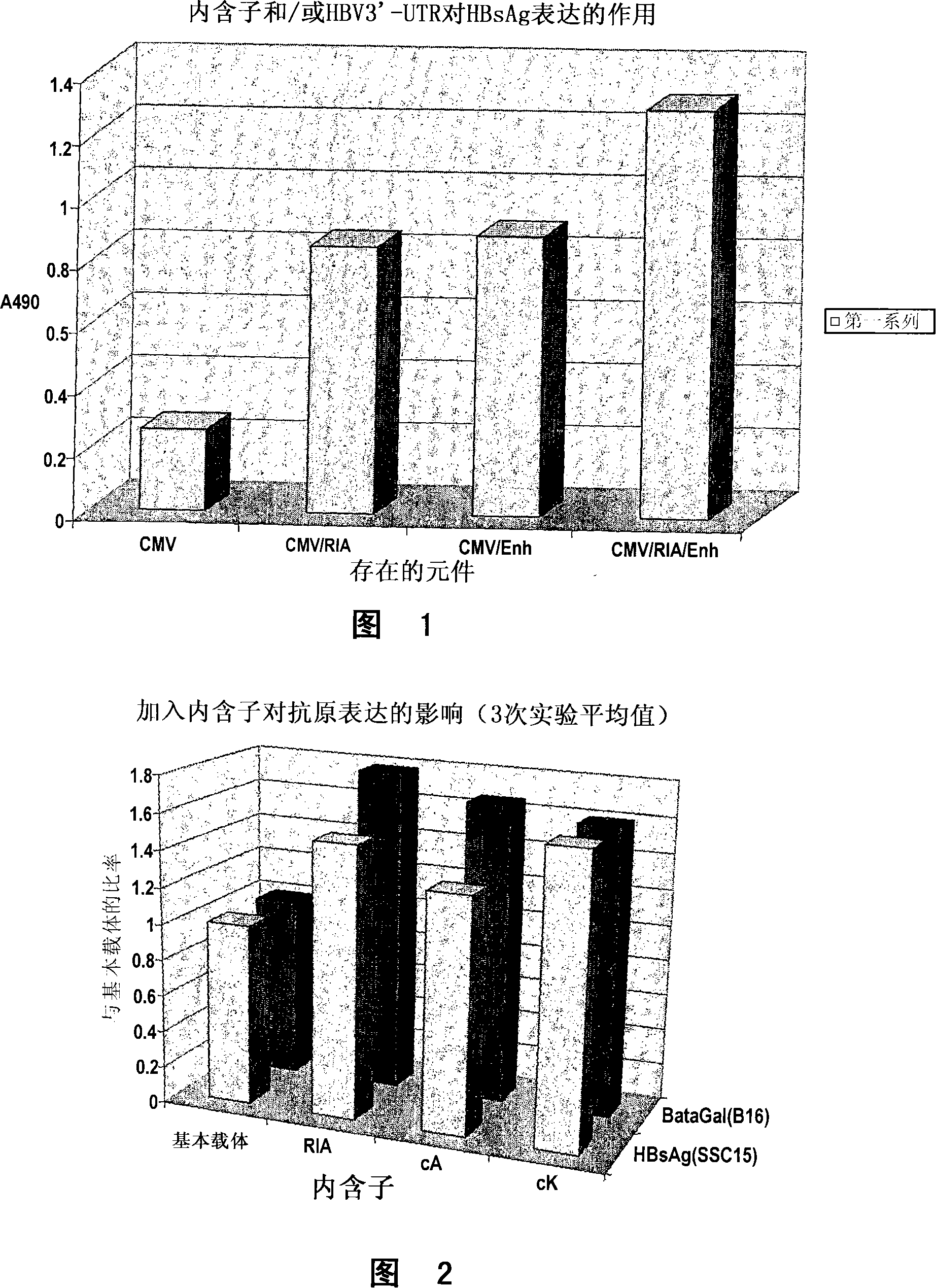
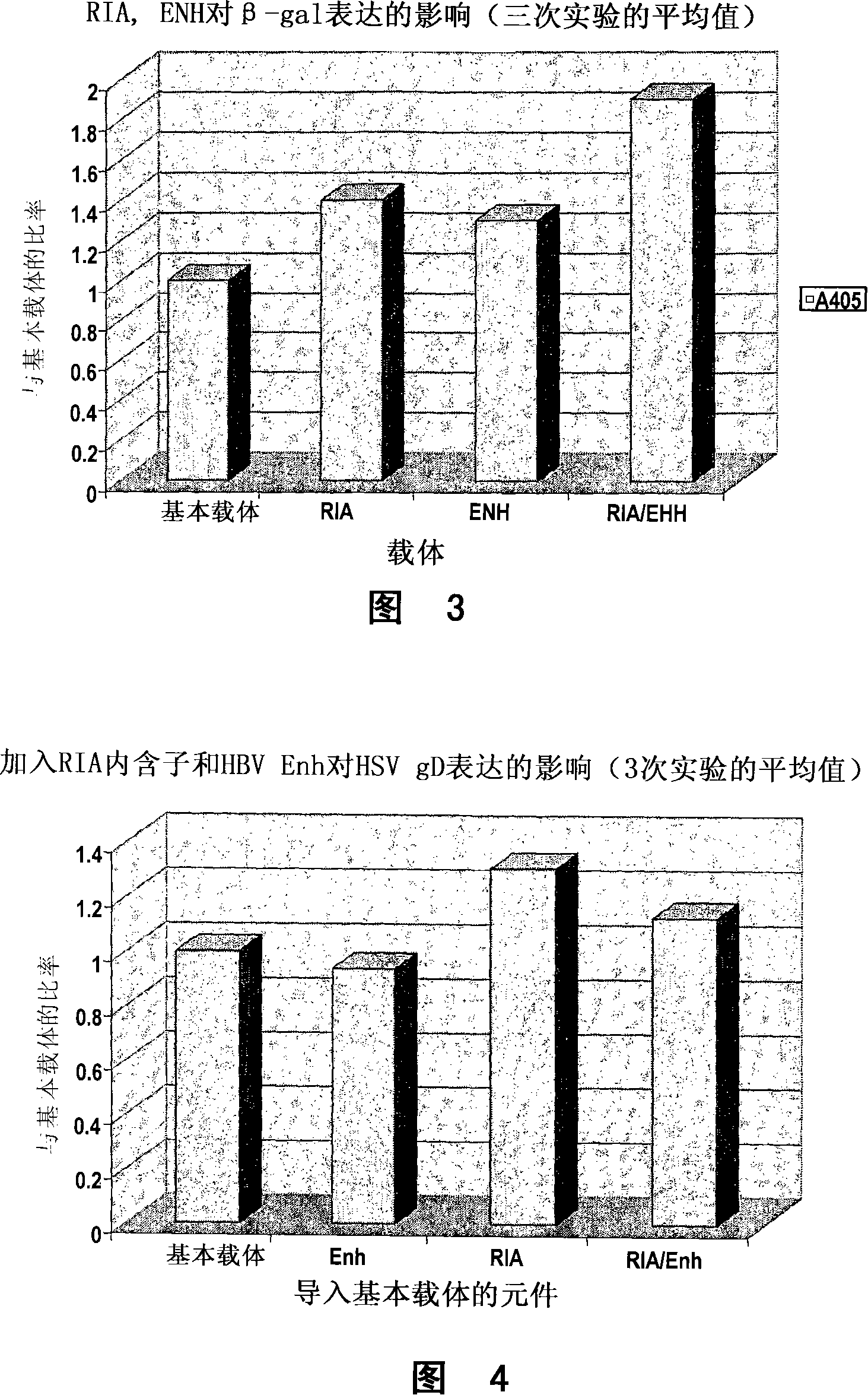
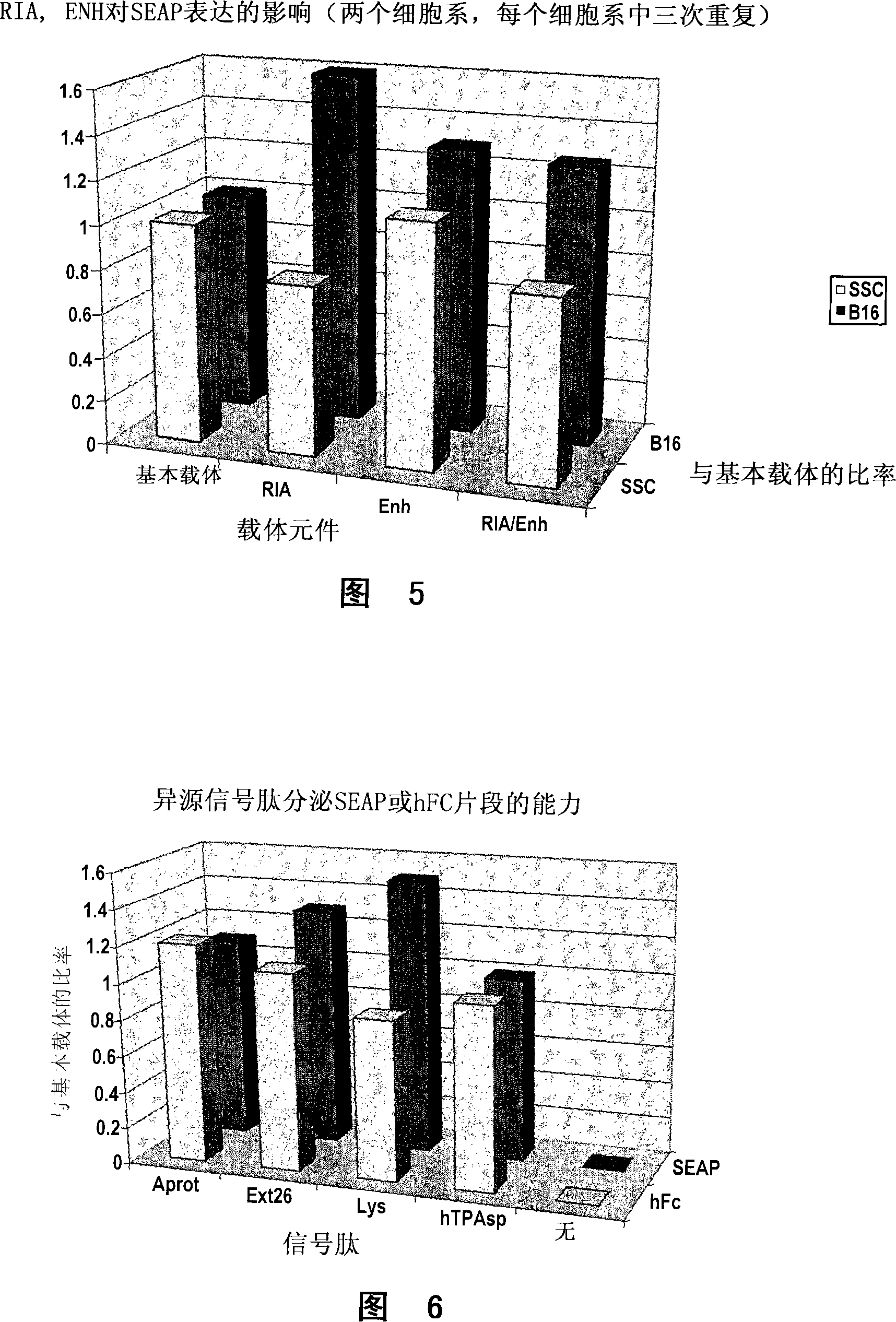
Nucleic acid constructs
A technology of nucleic acid constructs and constructs, applied in the fields of molecular biology and immunology, can solve problems such as the reduction of the expression level of early promoters of hCMV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0520] Embodiment 1. Construction of hepatitis B virus surface antigen (HBsAg) carrier series
[0521] Many plasmid expression vectors expressing HBsAg have been constructed.
[0522] raw material
[0523] (i) pWRG7128 (Roy, M, et. al. Vaccine (2001) 19 :764-778), which contains the hCMV immediate early promoter sequence, the first exon, the first intron and part of the second exon of the hCMV major immediate early gene, with flanking regions (derived from HBV preS2 5' UTR sequence and 3' post-transcriptional response element) HBsAg coding sequence and bovine growth hormone polyadenylation region (BGHpA).
[0524] (ii) pJV7284, a derivative of pWRG7128, which replaces BGHpA with the rabbit globin polyadenylation region (RBGpA).
[0525] (a) pPJV7384 (CMV (intronless), HBV preS2 5'UTR and RBGpA)
[0526] Using standard conditions, pWRG7128 was amplified with JF93 (SEQ ID NO: 15) and F110 (SEQ ID NO: 16), Sal 1 and BamH 1 cuts to isolate the CMV promoter, exon 1 and pa...
Embodiment 2
[0534] Embodiment 2. Construction of herpes simplex virus glycoprotein D antigen (HSVgD) vector series
[0535] A number of plasmid expression vectors expressing HSV gD have been constructed.
[0536] raw material
[0537] (a) In pPJV7334, a derivative of pWRG7284 (pPJV 7284), the HBsAg coding sequence was replaced by in-frame Nhe1 immediately downstream of the ATG start codon, followed by a stuffer fragment with BamH1, followed by 5 of HBC Enh (enhancer). 'end.
[0538] (b) pWRG7202, a derivative of pGem3Z (Promega) with a stuffer fragment allowing for the fusion of the coding sequence to the human tissue plasminogen activator (TPA) signal peptide downstream of the Nhe1 site.
[0539] (a) pPJV7392 (CMV (native intron), HBsAg 3'UTR, HBsAg 5'UTR and RBGpA)
[0540]Use primer DS1 (SEQ ID NO: 21) and DA1 (SEQ ID NO: 22) from virus DNA stock (AdvancedBiotech, Inc, Columbia, MD) PCR amplifies the coding region of HSV2gD, and cut with Nhe 1 and EcoR1, with An insert is for...
Embodiment 3
[0550] Example 3. Construction of Flu M2 antigen carrier series
[0551] (a) pPJV7450 (CMV (intronless), HBsAg 5'UTR and RBGpA)
[0552] The Flu M2 coding region was PCR amplified from plasmid pFL-M2 (Joel Haynes, pPJV) using primers JF301 (SEQ ID NO:23) and JF302 (SEQ ID NO:24) and cut with Nhe1 and Bgl2 to create an insert fragment. Nhe 1 and Bgl 2 cut pPJV7401 to form a vector fragment into which the insert was ligated, resulting in pPJV7450.
[0553] (b) pPJV7452 (CMV (intronless), HBsAg 3'UTR, HBsAg 5'UTR and RBGpA)
[0554] The 3'UTR fragment was PCR amplified from pPJV7389 using primers JF84 (SEQ ID NO: 25) and JF225 (SEQ ID NO: 26), Bsp120I cut, T4 DNA polymerase fill in, Bgl 2 linker (cat#1036, New England Biolabs )connect. This fragment was then cut with Bgl2 and EcoR1 to isolate the insert containing the HBsAg 3'UTR and RBGpA region. Bgl2 and EcoR1 cut pPJV7450 to form a vector fragment into which the insert is ligated, resulting in pPJV7452.
[0555] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com