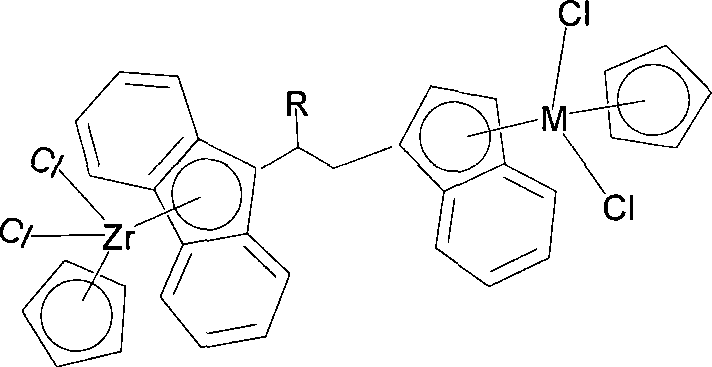
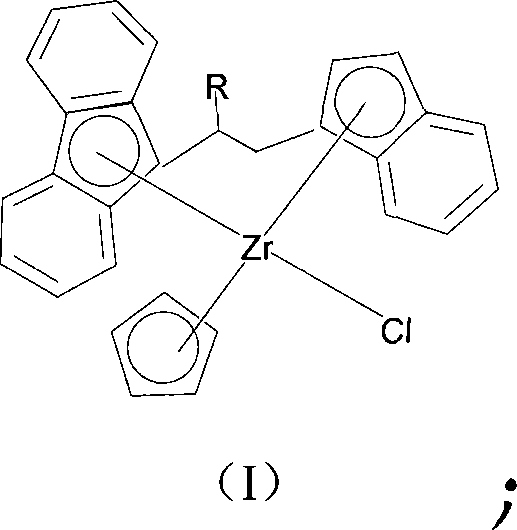
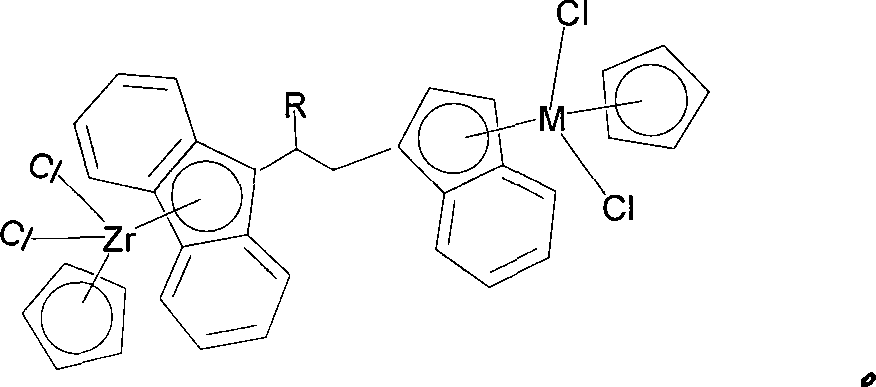
Bridged metallocene Zr-tombarthite catalyst and preparation method thereof
A metallocene compound and rare earth technology, applied in the field of polyolefins, can solve the problems of residence, catalytic mechanism, and the structure-activity relationship between catalyst structure and catalytic activity without further research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of compound (I) is as follows:
[0033] 1) Synthesis of ω-alkenyl epoxides
[0034] In organic solvents, the general formula is (wherein n is an integer of 1-10, preferably, n is an integer of 1-5; more preferably, n is 1, 3 or 5) of ω-diene and m-chloroperoxybenzoic acid in a molar ratio of 1: The ratio of 0.6 to 0.8 is reacted in an organic solvent, as shown in the following formula, to obtain 1,2-epoxy-alkyl ω-ene.
[0035]
[0036] The organic solvent is selected from dichloromethane, toluene, tetrahydrofuran.
[0037] 2)C 13 h 8 -R (R = alkenyl or substituted alkenyl) type of ligand synthetic synthesis method is shown in the following formula:
[0038]
[0039] Step 1) The obtained epoxide is reacted with an equimolar number of fluorene lithium to obtain the corresponding alcoholate, the reaction temperature is -90~-60°C, and the reaction time is 2-8 hours; then hydrolyzed to obtain 1-fluorene-ω- Alkyl-2-ols. Hydrolysis can be p...
Embodiment 1
[0061] Embodiment 1: the synthesis of binuclear catalyst A
[0062] Step 1: Synthesis of ω-alkenyl epoxides
[0063] 1,5-Hexadiene is reacted with m-chloroperoxybenzoic acid, as shown in the following formula, to obtain 1,2-epoxy-alkyl ω-ene epoxy compound.
[0064]
[0065] At room temperature, a solution of 13.75g (79.6mmol) m-chloroperbenzoic acid in 250ml dichloromethane was slowly dropped into a solution of 9g (110mmol) 1,5-hexadiene in 200ml dichloromethane, and the mixture was stirred overnight. The precipitate was removed by filtration, followed by 2M NaHCO 3 , 2N KOH and water, the organic layer was dried with anhydrous sodium sulfate, the solvent was removed in vacuo, and the residue was distilled to obtain an epoxy compound with a yield of 60-80%. The product was a colorless liquid, b.p.119-121°C.
[0066] 1 H NMR (CDCl3, 25°C): 5.68 (m, 1H, =CH), 4.83 (m, 1H, =CH 2 ), 4.83 (m, 1H, =CH 2 ), 2.73 (ABMX 2 , 1H, CH), 2.54 (ABM, 1H, CH 2 ), 2.27 (ABM, 1H, CH ...
Embodiment 2
[0077] The compound obtained in Example 2 undergoes a nucleophilic substitution reaction with indene lithium to obtain a C2 bridged ligand.
[0078]
[0079] At -78°C, 61mmol indene lithium in 100ml ether solution was mixed with 1-fluorene-hexa-5-diene-2-methanesulfonate, stirred for 24 hours, hydrolyzed with 50ml water, and the organic layer was washed with anhydrous sodium sulfate Dry and remove solvent in vacuo. The residue was dissolved with pentane and passed through a silica gel column. Crystallization in pentane at -18°C yield 40-50%. 6-(9-fluorene)-5-(1-indene)-1-hexene was obtained: GC: 2844s; MS: m / e362 (M + ); Its NMR data are as follows: 1 H NMR (CDCl 3 , 25°C): 7.71-7.23 (12H), 6.95 (m, 1H), 6.55 (m, 1H), 5.65 (m, 1H, =CH), 4.90 (m, 1H, =CH 2 ), 4.90 (m, 1H, =CH 2 ), 4.18(m), 3.95(m), 3.80(m, 1H, CH), 3.68(s), 3.56(s), 3.33(s, 1H, CH), 2.44(m, 1H, CH), 2.13 (m, 2H, CH 2 ), 1.85 (m, 2H, CH 2 ), 1.51 (m, 2H, CH 2 );
[0080] 13 C NMR (CDCl 3 , 25°C):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com