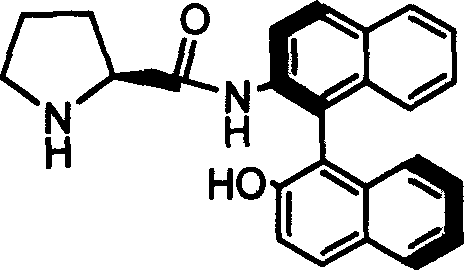
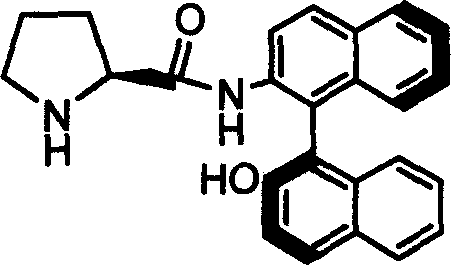
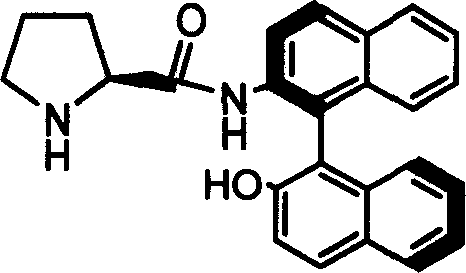
Organic small molecule catalyst of aqueous phase asymmetric direct aldol condensation reaction, and preparing method and use thereof
An aldol condensation reaction and catalyst technology, applied in the field of organic small molecule chiral catalysts, can solve the problems of being unfriendly to the environment, unsatisfactory results, and the catalyst cannot simultaneously obtain high yields, etc., and achieve the effect of high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation and resolution of N-benzyloxycarbonyl-protected catalyst precursors
[0026] Dissolve 3.98 g of L-form proline protected by benzyloxycarbonyl and 1.62 g of triethylamine in 30 mL of tetrahydrofuran solution, and cool to 0 degrees Celsius in an ice-water bath. Under the condition of stirring, 0.88 g of ethyl chloroformate was added dropwise, and stirring was continued for 30 minutes after the dropwise addition was completed. 4.56 g of racemic binaphtholamine was dissolved in 100 mL of THF, and added dropwise to the reaction mixture. After the dropwise addition, the reaction was continued at 0°C for 1 hour, and then at room temperature for 24 hours. After the reaction stopped, the solid was filtered off, and the solvent was distilled off under reduced pressure on a rotary evaporator. The resulting substance was subjected to flash column chromatography with silica gel H, and the developing solvent was petroleum ether: ethyl acetate=2:1, and the configuration ...
Embodiment 2
[0028] Preparation of Catalyst 1a
[0029] Add 2g of Z-1a obtained from Example 1, 0.4g of 10% Pd / C and 60ml of methanol into a 100ml two-necked flask, feed 1atm of hydrogen under reflux at 40°C, and stir for 1 hour. After the reaction was finished, the solid was filtered off, and the resulting solution was evaporated to dryness and recrystallized with dichloromethane to obtain the catalyst 1a.
[0030] Melting point: 240-241℃; specific rotation value: [] 20 D =-23.46 (c=0.5, THF); NMR analysis: (600MHz, CDCl 3 ) (ppm) 1.22-1.28 (m, 2H) 1.46-1.50 (m, 1H) 1.67-1.72 (m, 1H) 1.83-1.88 (m, 1H) 2.33-2.35 (m, 1H) 3.56-3.58 (m, 1H) 5.29 (s, 1H) 7.02-7.04 (d, 1H, J = 8.5Hz) 7.22-7.25 (m, 1H) 7.29-7.39 (m, 3H) 7.38-7.39 (d, 1H, J = 8.88Hz) 7.42-7.44 (m, 1H) 7.87-7.88 (d, 1H, J = 8.2Hz) 7.92-7.97 (dd, 2H, J = 8.2, J = 8.88) 8.04-8.06 (d, 1H, J = 8.9Hz) 8.80-8.82 (d, 1H, J=8.9Hz) 9.76 (s, 1H); 13 CNMR (600MHz, CDCl 3 )(ppm)25.36,30.68,46.23,60.70,113.62,116.90,117.74,119.82,123.82...
Embodiment 3
[0032] Preparation of Catalyst 1b
[0033] Add 2g of Z-1b obtained from Example 1, 0.4g of 10% Pd / C and 60ml of methanol into a 100ml two-neck flask, feed 1atm of hydrogen under reflux at 40°C, and stir for 1 hour. After the reaction was finished, the solid was filtered off, and the resulting solution was evaporated to dryness and recrystallized with a mixed solvent of petroleum ether and tetrahydrofuran to obtain catalyst 1b.
[0034] Melting point: 236-237℃; Specific rotation value: [] 20 D =-7.88 (c=0.5, THF); NMR analysis: 1 HNMR (600MHz, CDCl 3 ) (ppm) 1.21-1.23 (m, 1H) 1.36-1.46 (m, 1H) 1.48-1.52 (m, 1H) 1.80-1.85 (m, 1H) 1.91-1.97 (m, 1H) 2.24-2.27 (m, 1H) 2.57-2.61 (m, 1H) 3.53-3.56 (dd, 1H, J = 9.4, J = 4.1) 5.29 (s, 1H) 7.01-7.03 (d, 1H, J = 8.5) 7.23-7.28 (m, ( d, 1H, J=8.9) 8.66-8.67 (d, 1H, J=8.9); 13 CNMR (600MHz, CDCl 3 )(ppm)25.69,30.71,46.69,60.68,113.88,117.78,118.18,120.45,123.70,124.36,125.25,127.09,127.18,128.23,128.31,129.35,130.26,130.65,131.19,133...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com