Brilliant mycomycin elaioplast preparation and its making method
A technology of liposome preparation and leucectin A, which is applied in liposome delivery, antiviral agents, pharmaceutical formulations, etc., can solve the problems of organic solvent allergy, limited clinical application, adverse reactions in human body, etc., and achieve improvement of liver function , enhance the detoxification function, increase the effect of bile permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
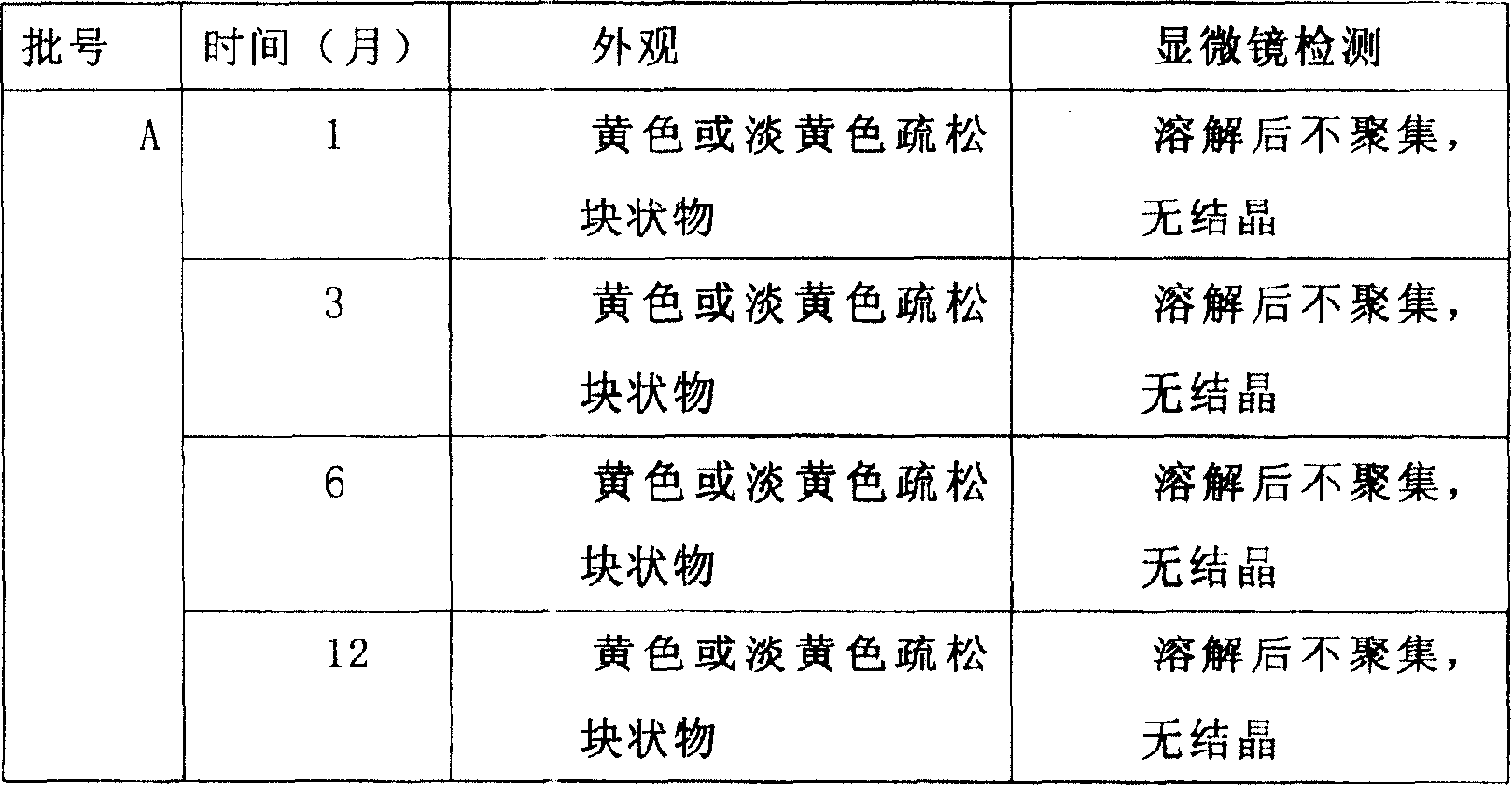
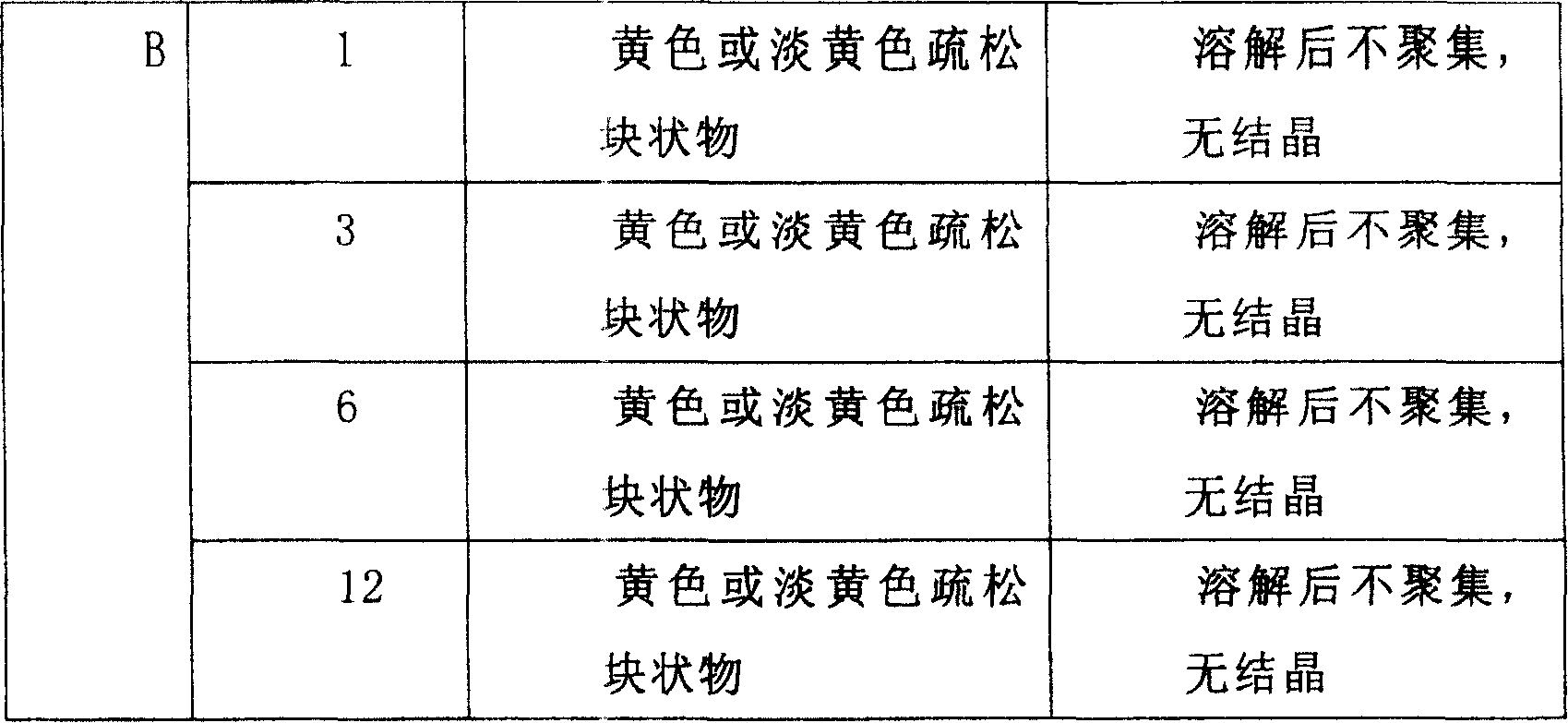
Image
Examples
Embodiment 1
[0029] The process steps of the preparation method of the leucovorin A liposome preparation for treating acute cholecystitis, chronic cholecystitis attack, chronic superficial gastritis and other diseases of the present invention are as follows: its formula is composed: in g / ml:
[0030] In the present invention, 5 g of Leucin A, 50 g of purified polyene phospholipid, and 8 g of cholesterol are placed in a round-bottomed flask, about 150 ml of ethanol is added to the above mixture to make it completely dissolved into a clear solution, and it is placed in a constant temperature water bath at 60° C. for rotary drying for 0.5 to 10 hours. Remove the solvent to form a film, and then perform vacuum drying treatment. Rotation speed: 30-100 rpm, vacuum drying: 4 hours, vacuum drying temperature: 20-60 °C, vacuum degree: 0.08-0.095Mpa; 1000ml of a solution containing 3g of aspartic acid, 20g of vitamin C, and 50g of mannitol dissolves the film, and ultrasonically treats it with an ultr...
Embodiment 2
[0032] The process steps of the preparation method of leucovorin A liposome preparation of the present invention are as follows: its formula is composed: in g / m1:
[0033] In the present invention, 10 g of Leucin A, 60 g of refined soybean lecithin and 10 g of cholesterol are placed in a round-bottomed flask, and about 180 ml of diethyl ether is added to the above mixture to completely dissolve into a clear solution, set in a constant temperature water bath at 55° C., and spin-dried for 0.5 to 10 hours. Remove the solvent to form a film, and then carry out vacuum drying treatment, rotation speed: 30-100 rpm, vacuum drying: 4 hours, vacuum drying temperature: 20-60 °C, vacuum degree: 0.08-0.095Mpa; 1000ml dissolving film of solution containing taurine 5g, sodium sulfite 15g, glucose 50g, pH value: 3~10; Homogenize 25 minutes~6 hours with high pressure homogenizing pump, homogenizing pump rotating speed: 500~10000 rev / min ; Filter and sterilize with 0.22um filter membrane, pack ...
Embodiment 3
[0035] The process steps of the preparation method of leucovorin A liposome preparation of the present invention are as follows: its formula is composed: calculated in g / ml as:
[0036]Weigh 20g of leucectin A, 70g of refined lecithin, and 12g of cholesterol in a round bottom flask, add about 200ml of chloroform to the above mixture to completely dissolve into a clear solution, place in a constant temperature water bath at 50°C, and spin dry for 0.5 to 10 hours to remove the solvent Form a film, and then carry out vacuum drying treatment, rotation speed: 30-100 rpm, vacuum drying: 4 hours, vacuum drying temperature: 20-60°C, vacuum degree: 0.08-0.095Mpa; 4g of ornithine, 10g of sodium pyrosulfite, 25g of mannitol, 25g of glucose in 1000ml to dissolve the film, pH value: 3-10; ultrasonic treatment with an ultrasonic instrument for 10 minutes to 3 hours, ultrasonic frequency: 20-30KHZ, ultrasonic power: 1000W , and then use a high-pressure homogenizer pump to homogenate for 15 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com