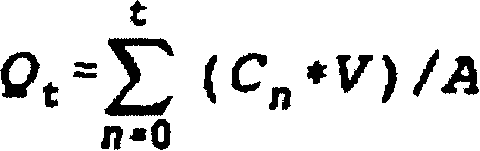Triacetin as a transdermal penetration enhancer
A technology of triacetin and patch, which is applied in anesthetics, drug combinations, cardiovascular system diseases, etc., and can solve the problems of poor bioavailability, low importance, and short half-life of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Oxybutynin free base (pK a = 10.3) is a strong basic drug for transdermal administration for antispasmodic and anticholinergic therapy. Matrix patches containing varying amounts of oxybutynin free base and penetration enhancer were prepared and tested as described above. The matrix system consists of 5-20% by weight oxybutynin free base and 0-20% by weight accelerator contained in a pharmaceutical grade acrylic copolymer binder.
[0053] Matrix formulations were prepared as follows. First, the solids content of the binder was determined by weighing a small amount of the binder solution in a weighed aluminum pan. The solvent was evaporated by drying in a convection oven at 80°C overnight, and the weight of the residue (dry binder) and the percent solid binder in solution were determined. After the solids content has been determined, a known weight of the acrylic copolymer adhesive solution is weighed in a glass bottle. The amount of binder in the solution was calcula...
Embodiment 2
[0067] According to the method of Example 1, various known accelerators were evaluated for their activity in promoting the transdermal absorption of oxybutynin free base, except that triacetin was replaced by these accelerators. The results of the in vitro skin absorption test are listed in Table 4.
[0068] Table 4
Accelerator
formula a
A / D / E(%W / W)
Q t (t = 24 hours)
(μg / cm 2 / t) b
J ss
(μg / cm 2 / hr) b
none
80 / 20 / 0
47.05±21.01
2.03±0.95
Sorbitan Monooleate
70 / 20 / 10
42.47±21.63
1.92±0.98
N-Methylpyrrolidone
60 / 20 / 20
54.36±1.98
2.42±0.97
70 / 20 / 10
24.29±8.73
1.25±0.41
Isopropyl myristate
70 / 20 / 10
48.26±13.08
2.05±0.54
glyceryl monooleate
70 / 20 / 10
52.78±8.25
2.25±0.32
[0069] a A = binder = TSR; D = drug = oxybutynin free base; E = accelerator = t...
Embodiment 3
[0073] Yantong Xikang is a weak alkaline anti-inflammatory, analgesic and antipyretic agent, its pK a is 6.3. According to the method of Example 1, the activity of triacetin in promoting the percutaneous absorption of piroxicam was evaluated, except that oxybutynin was replaced by piroxicam. The results are listed in Table 5.
[0074] table 5
Example number
formula a A / D / E(%W / W)
Q t (t=24 hours) (μg / cm 2 / t) b
1
99.75 / 0.25 / 0
0.56±0.30
99.25 / 0.25 / 0.5
0.58±0.07
[0075] 97.75 / 0.25 / 2.0
0.32±0.08
95.75 / 0.25 / 4.0
0.45±0.17
2
99.75 / 0.25 / 0
0.55±0.31
99.25 / 0.25 / 0.5
0.27±0.15
97.75 / 0.25 / 2.0
0.03±0.02
95.75 / 0.25 / 4.0
0.18±0.04
3
99.75 / 0.25 / 0
0.60±0.20
99.25 / 0.25 / 0.5
0.36±0.14
97.75 / 0.25 / 2.0
0.42±0.09
95.75 / 0.25 / 4.0
0.31±0.14
[0076] a A = Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com