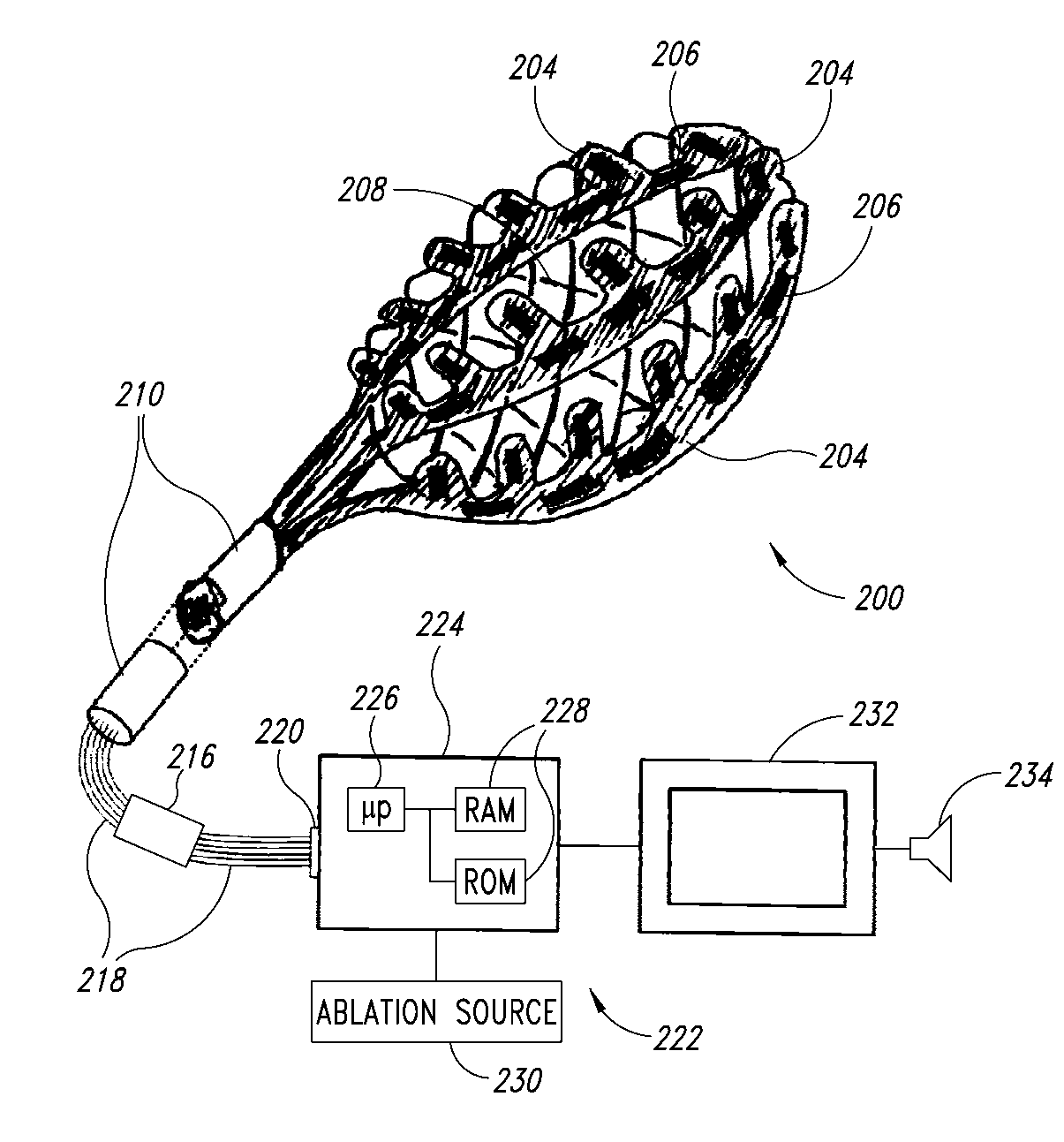
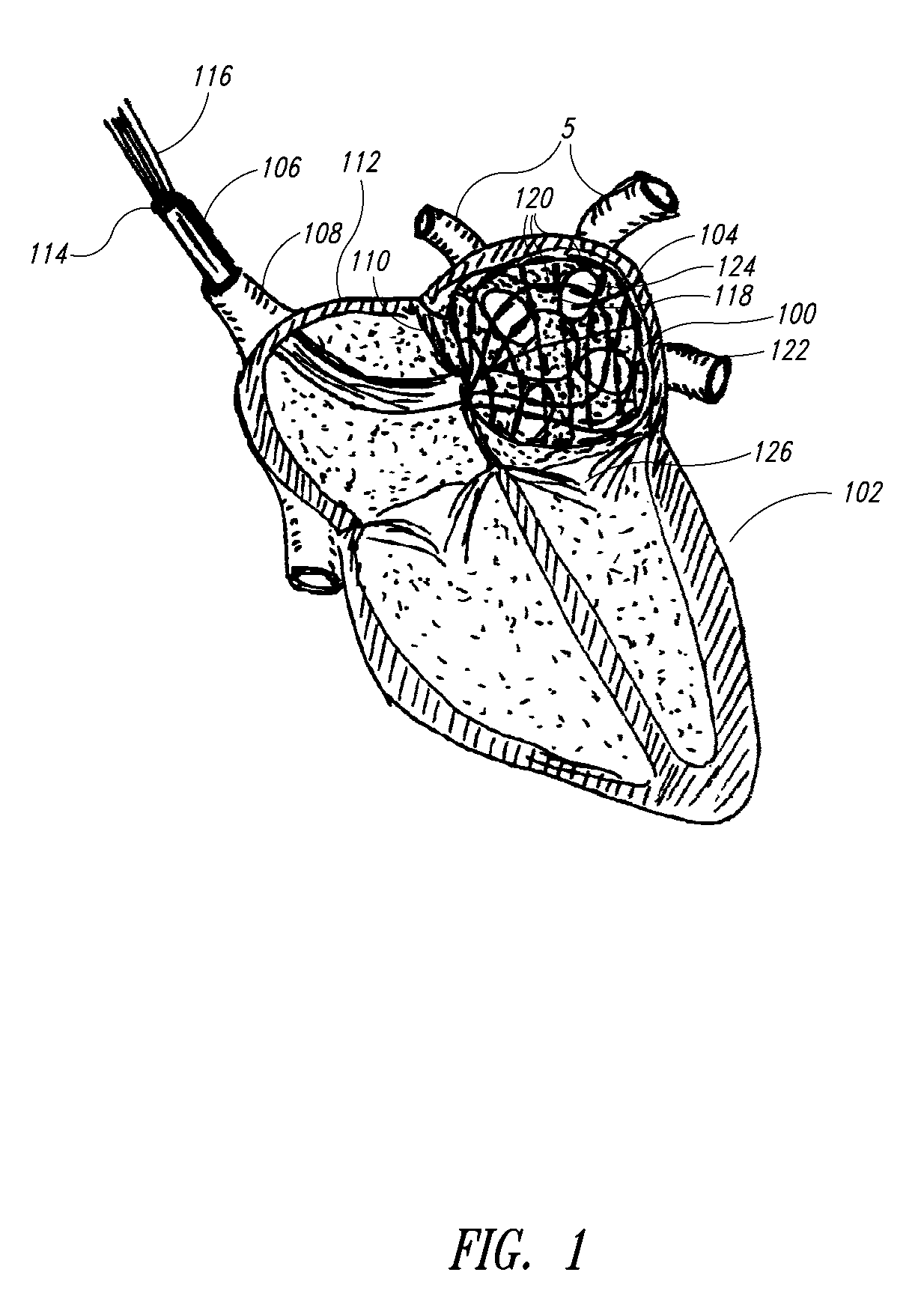
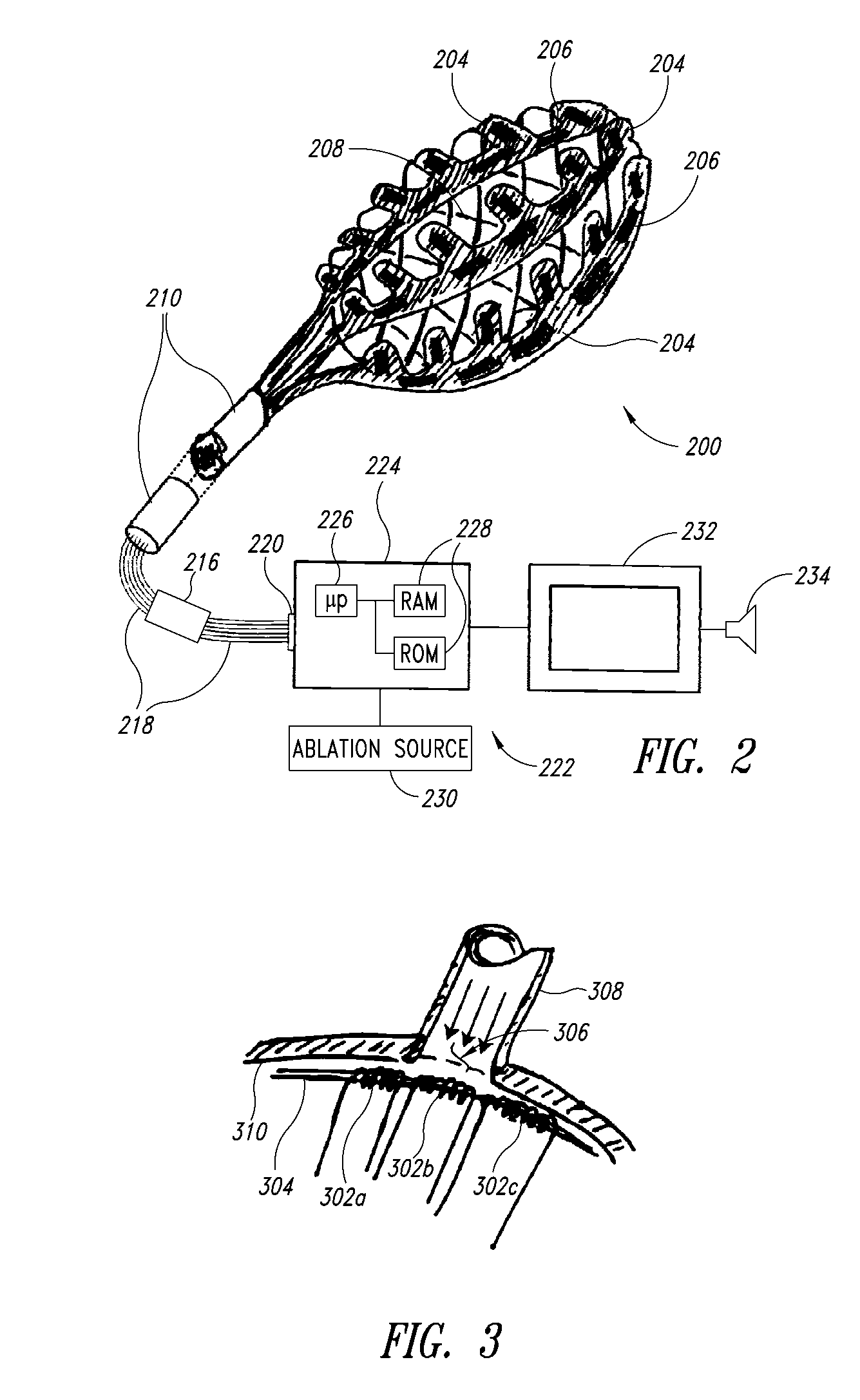
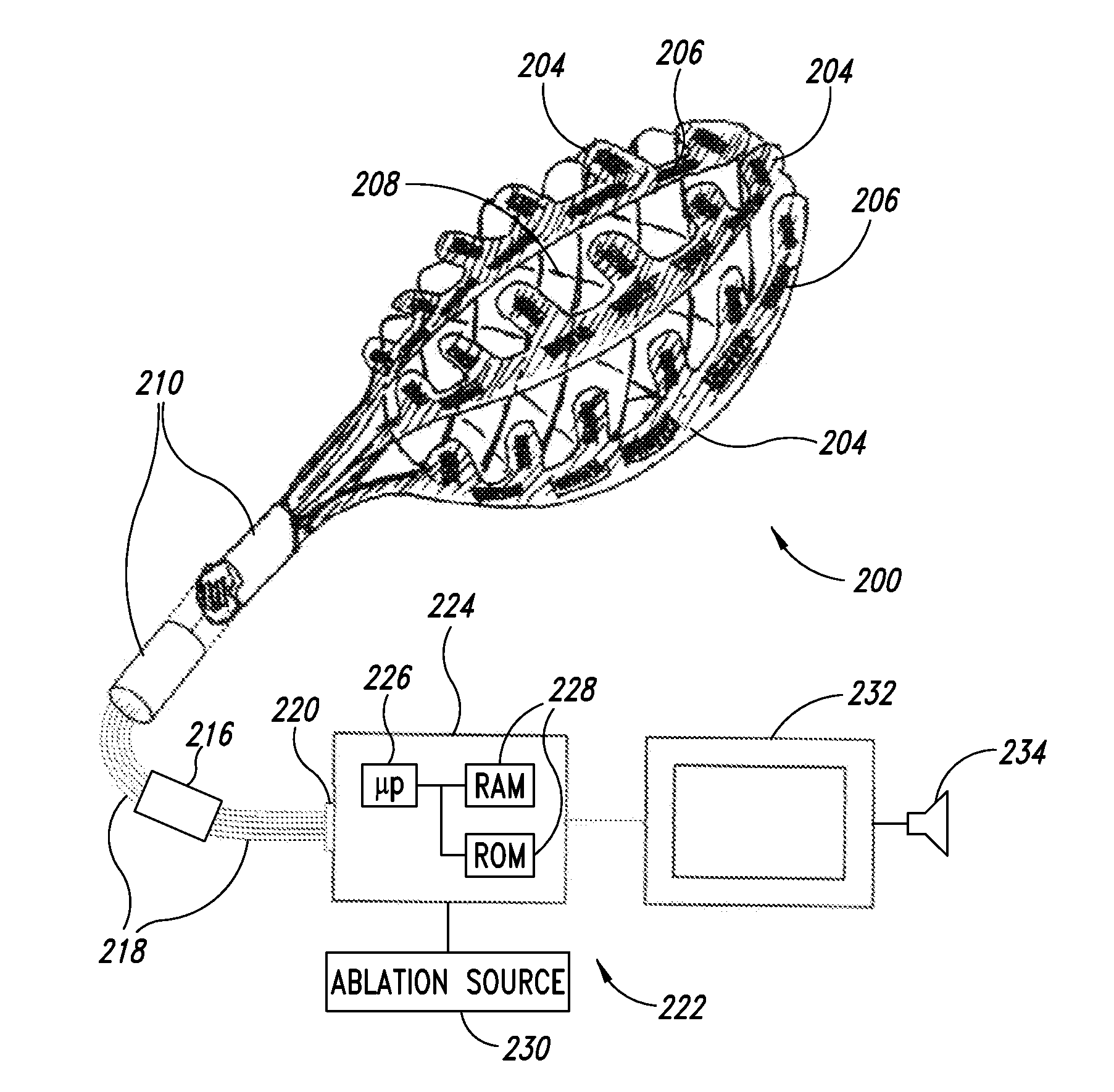
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34results about How to "Improve ablation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
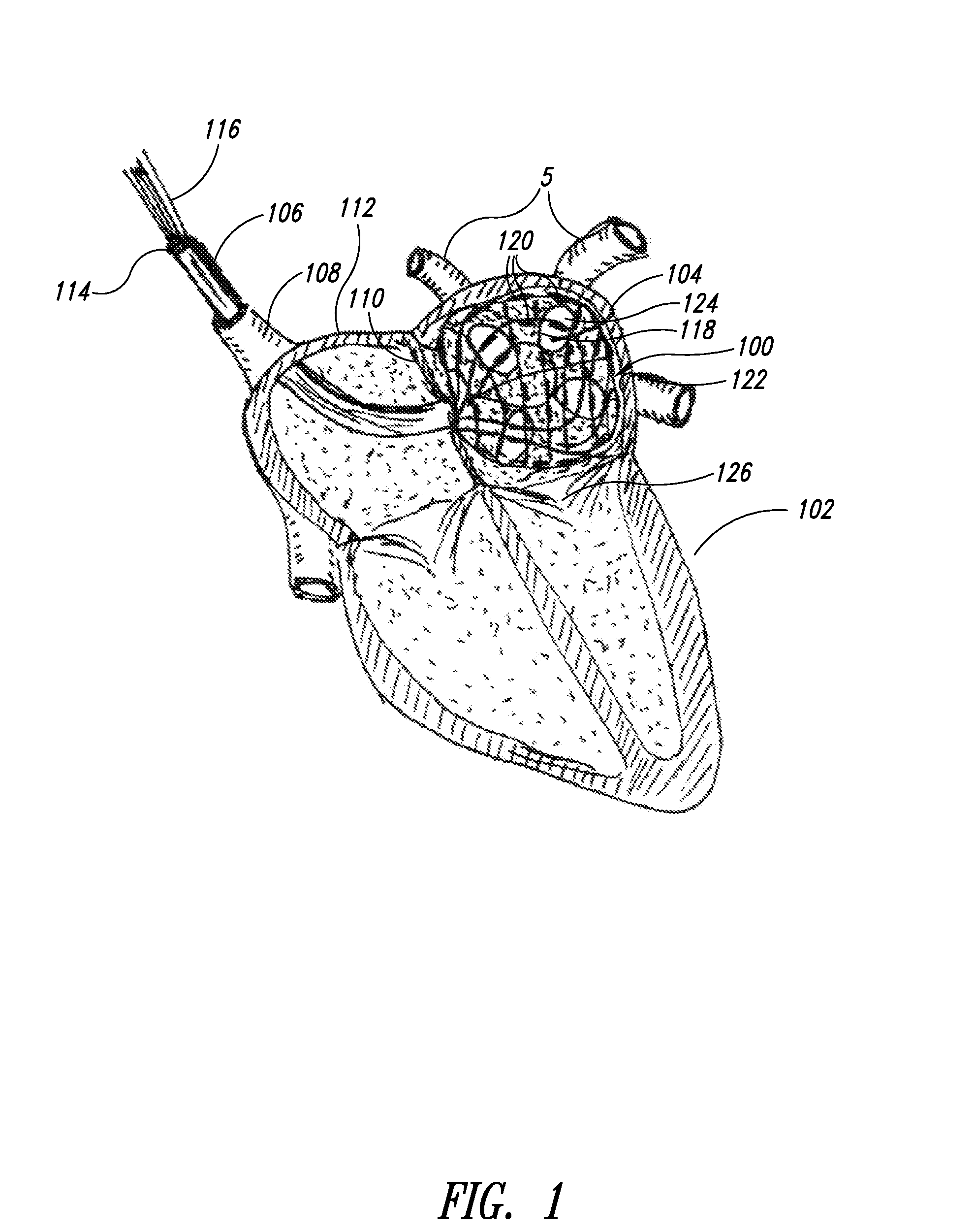
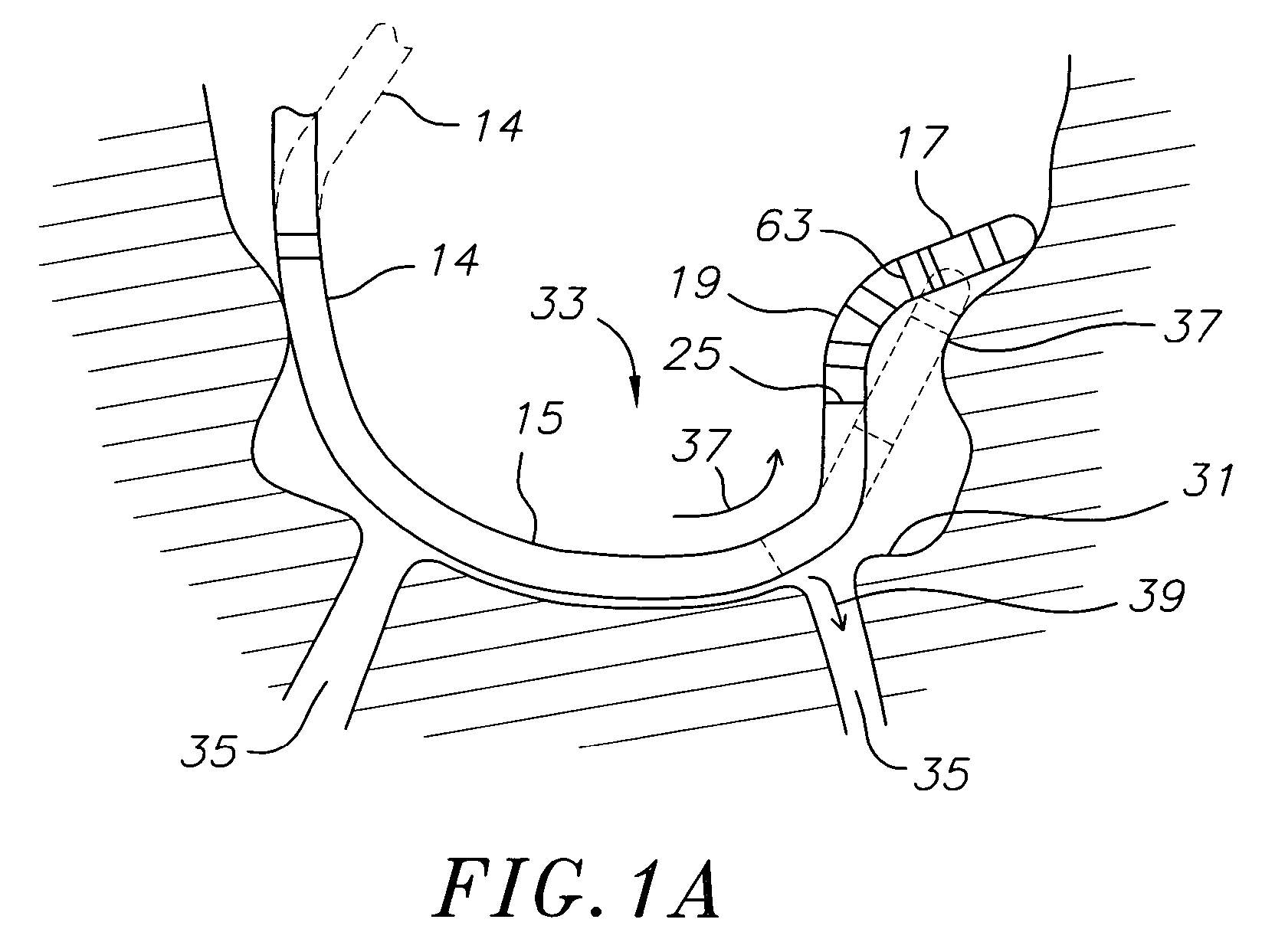
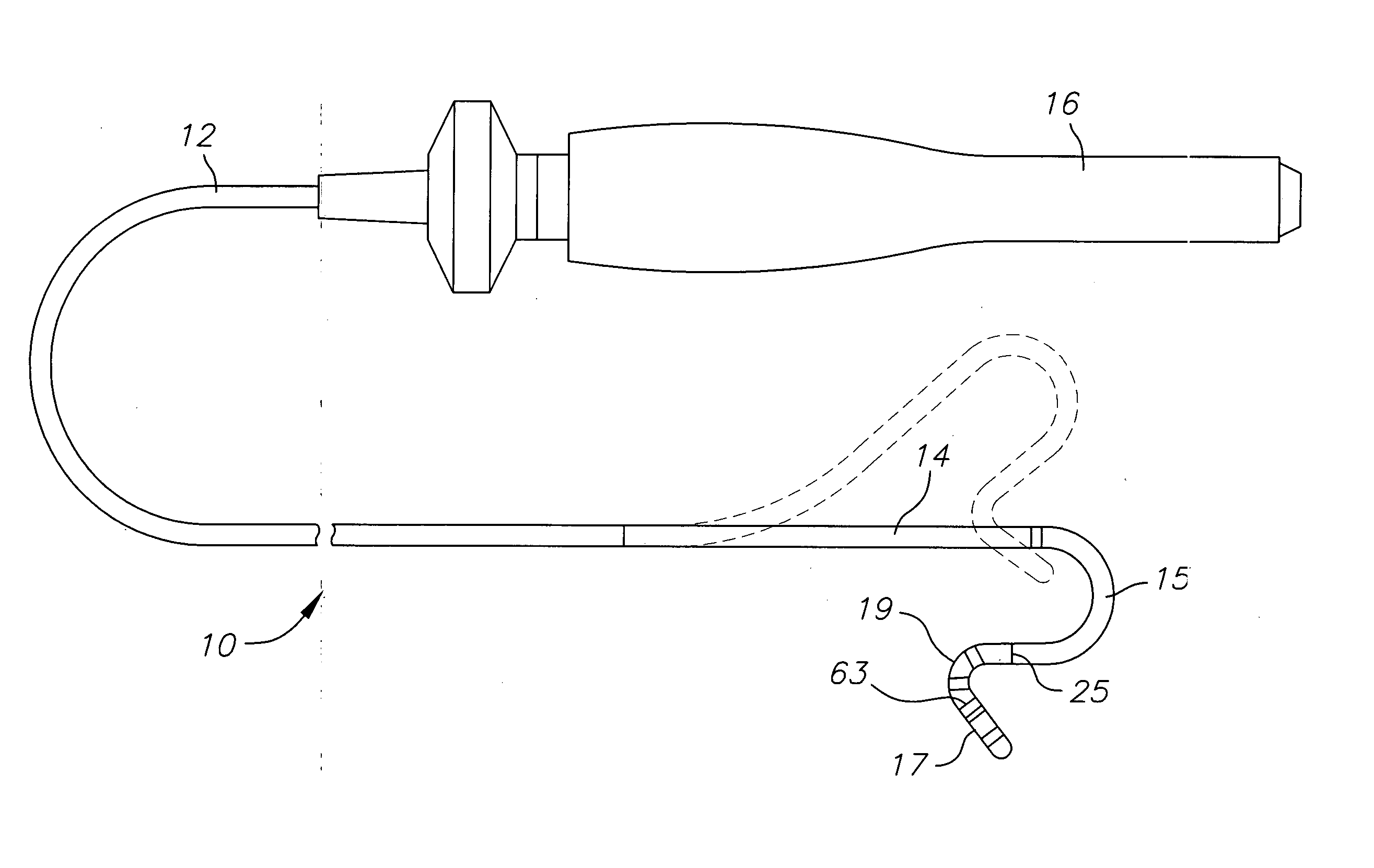
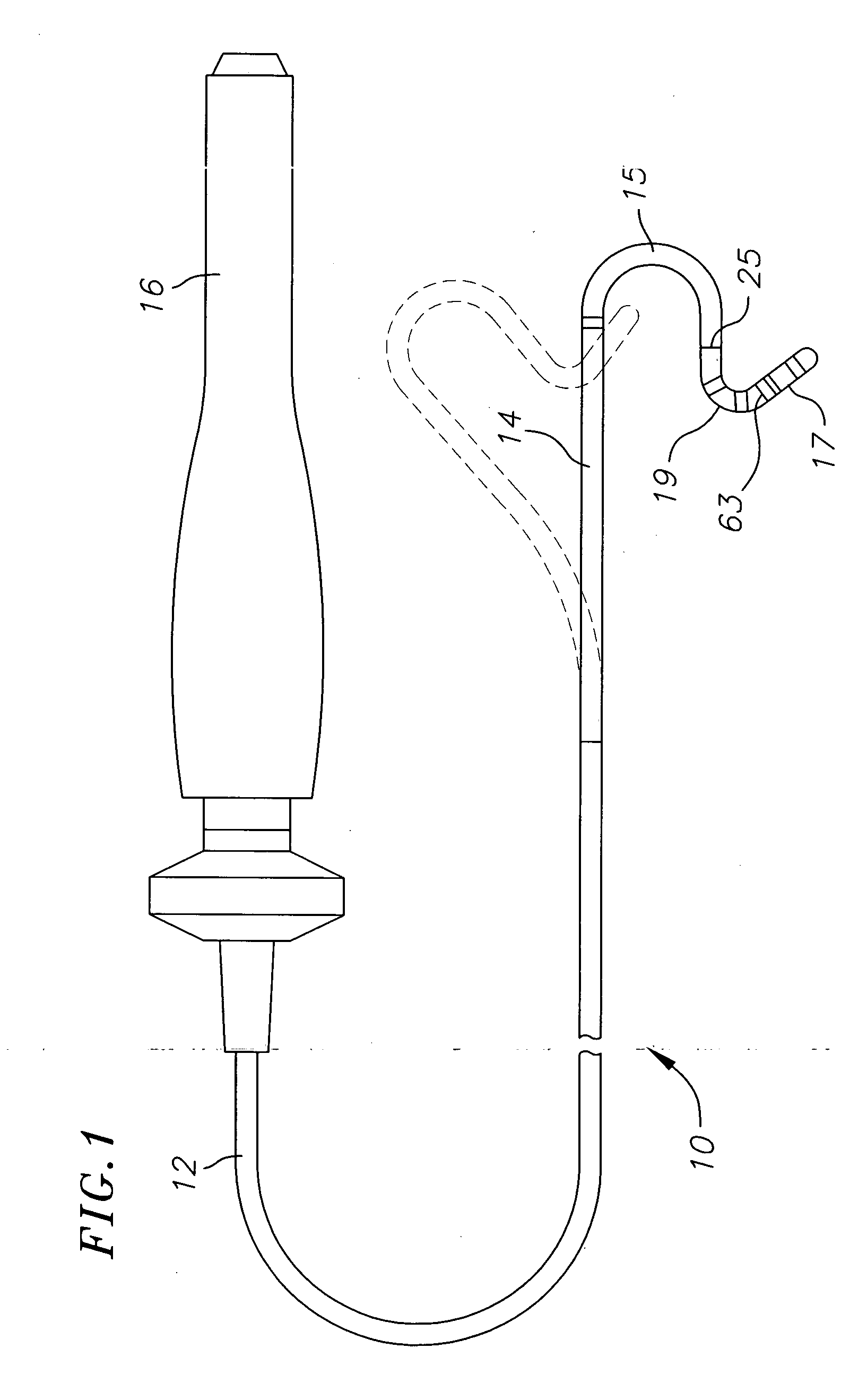
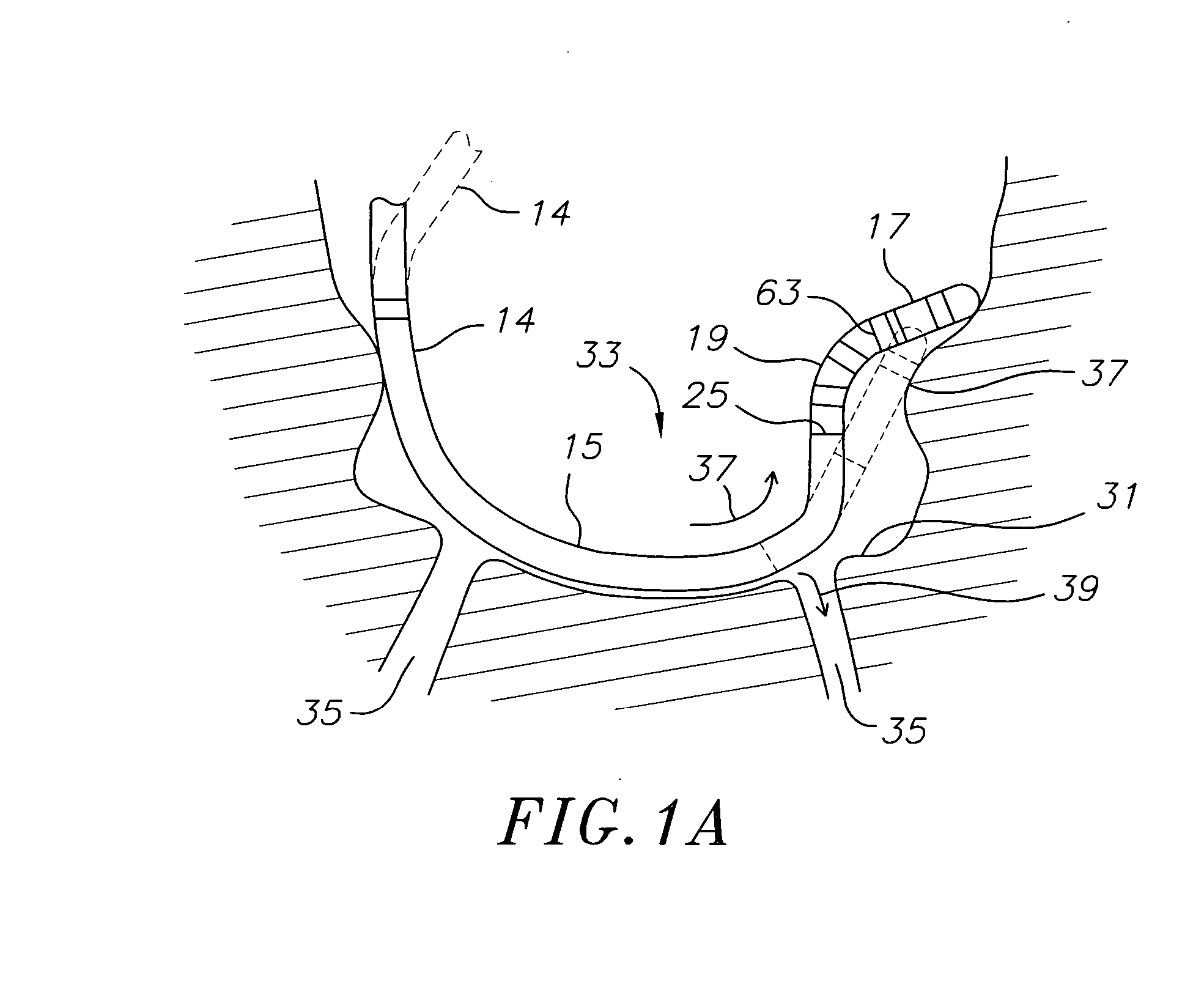
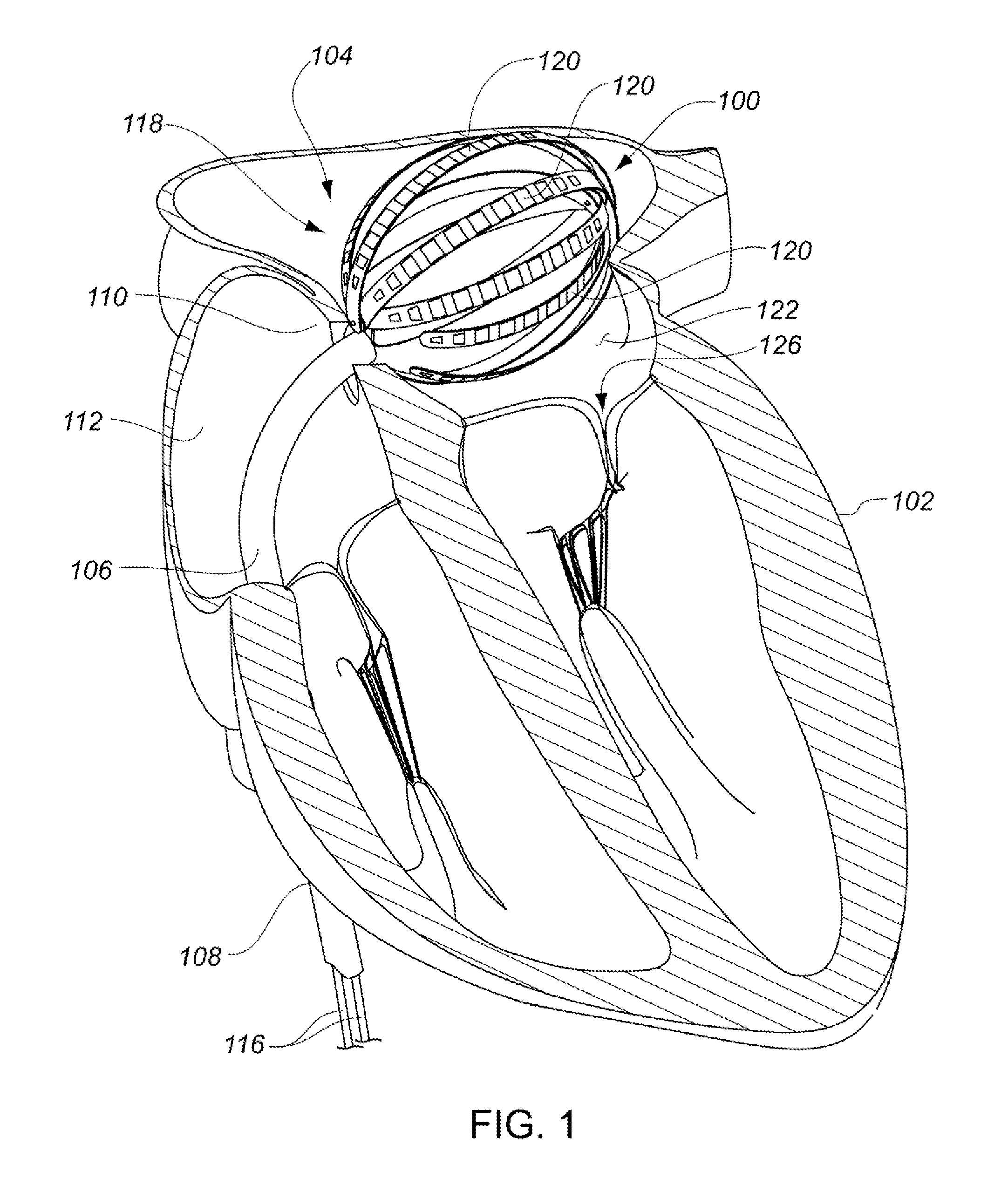
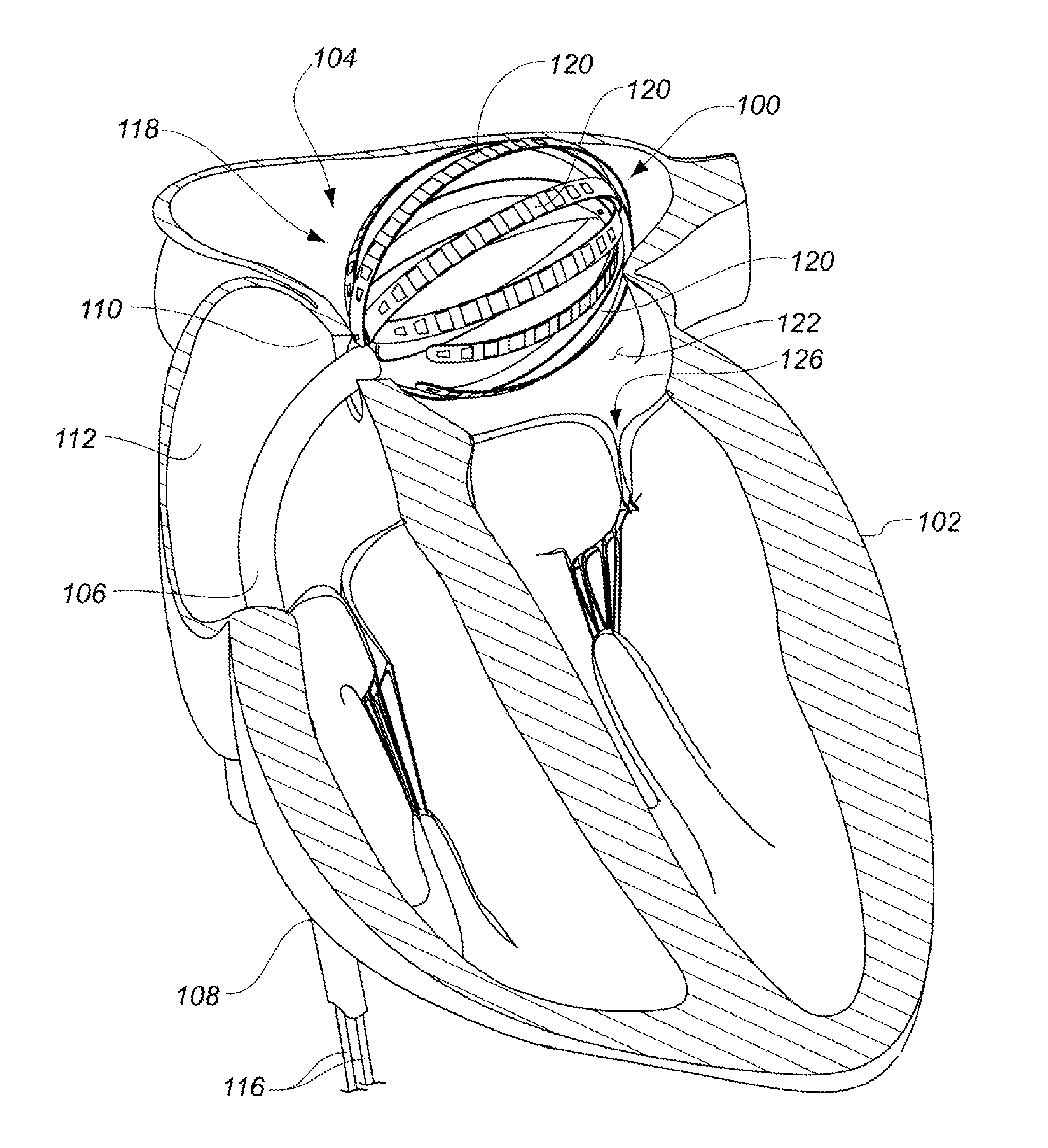
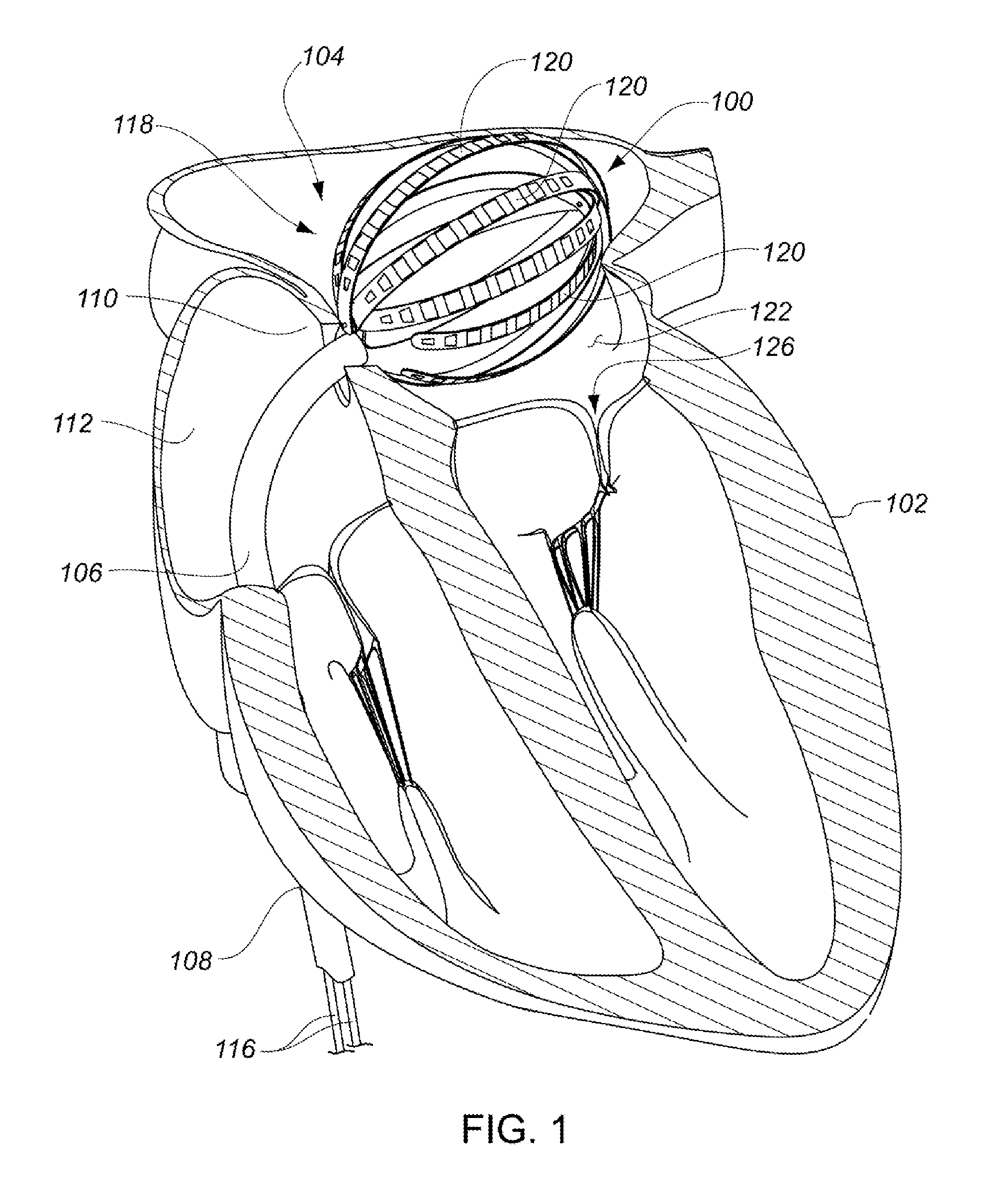
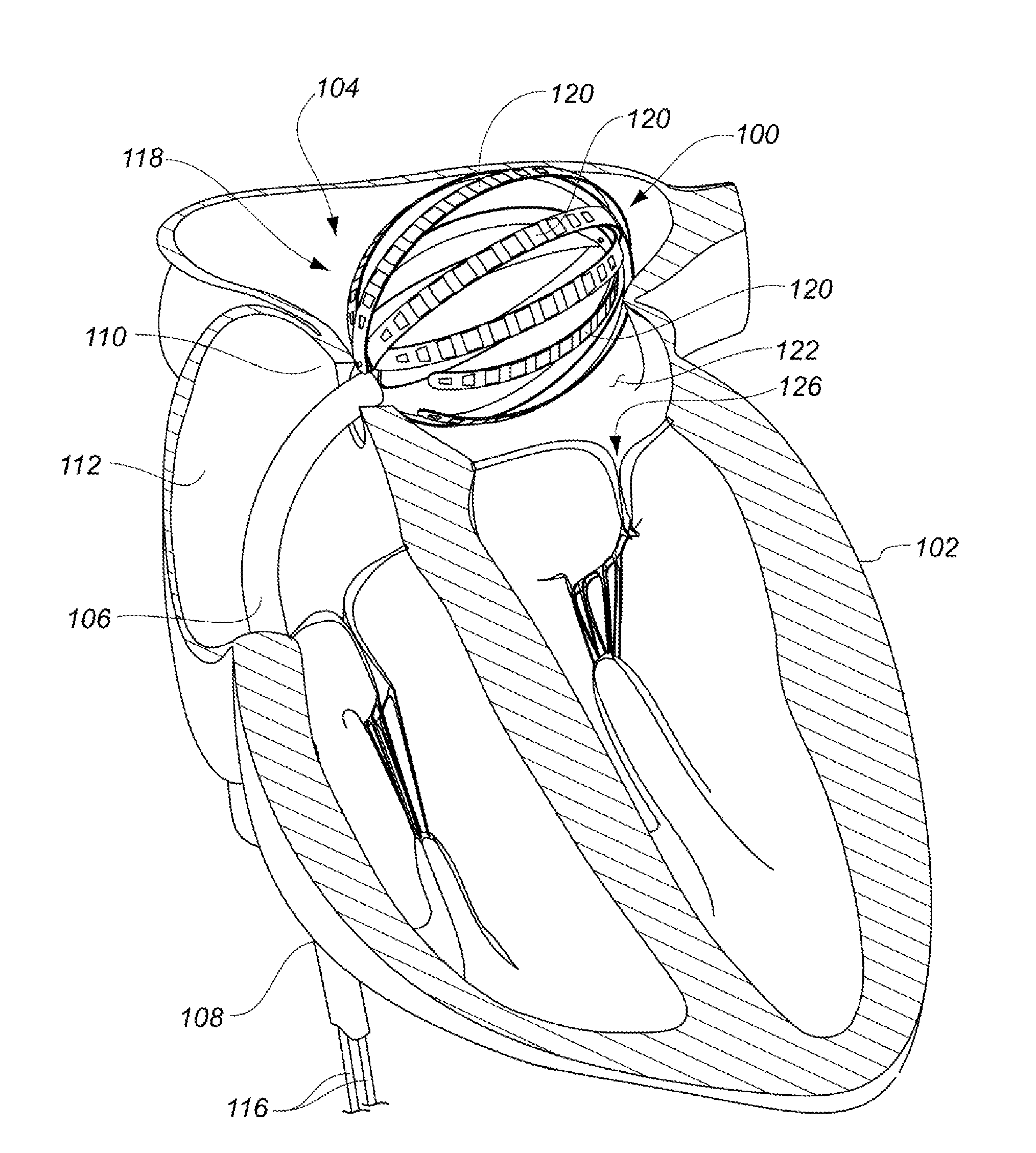
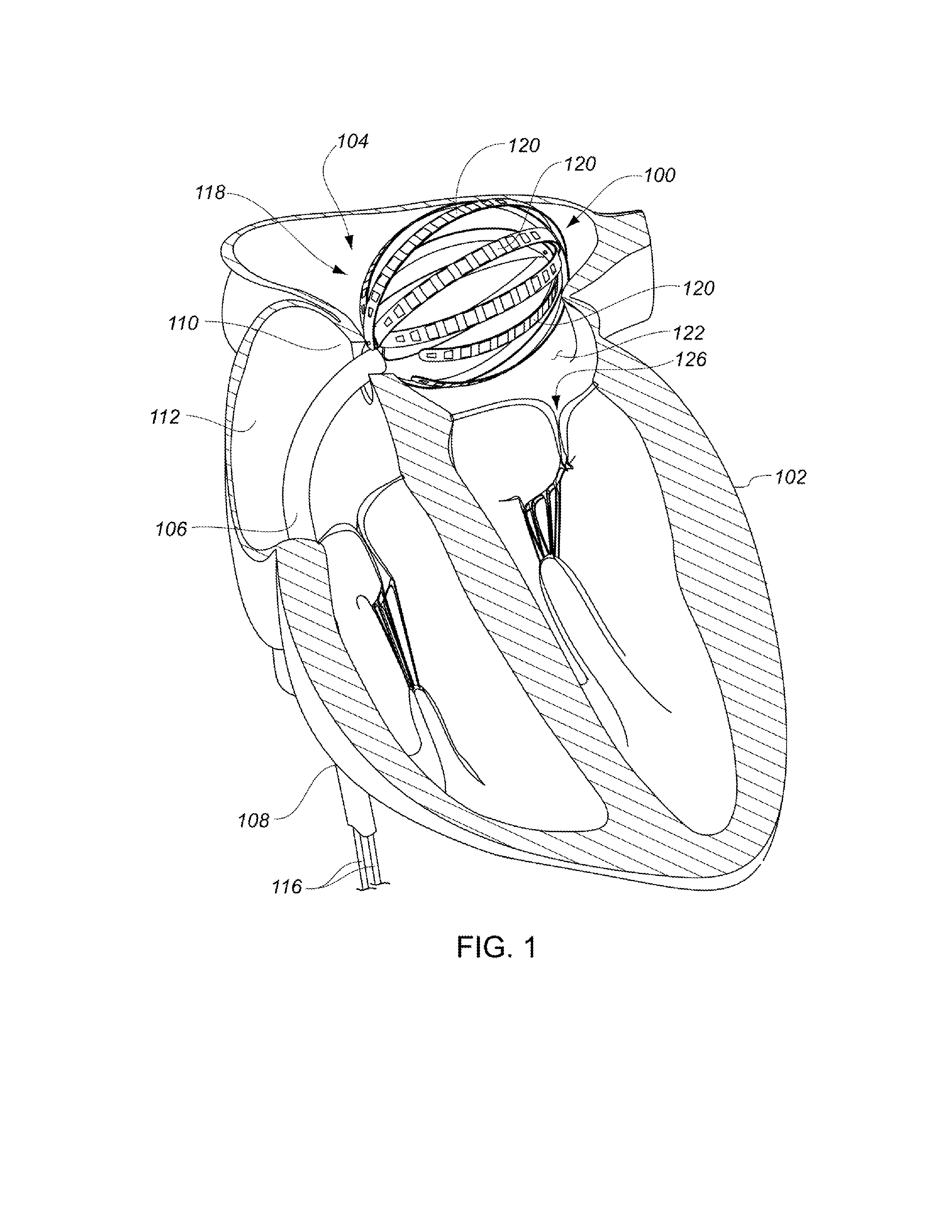
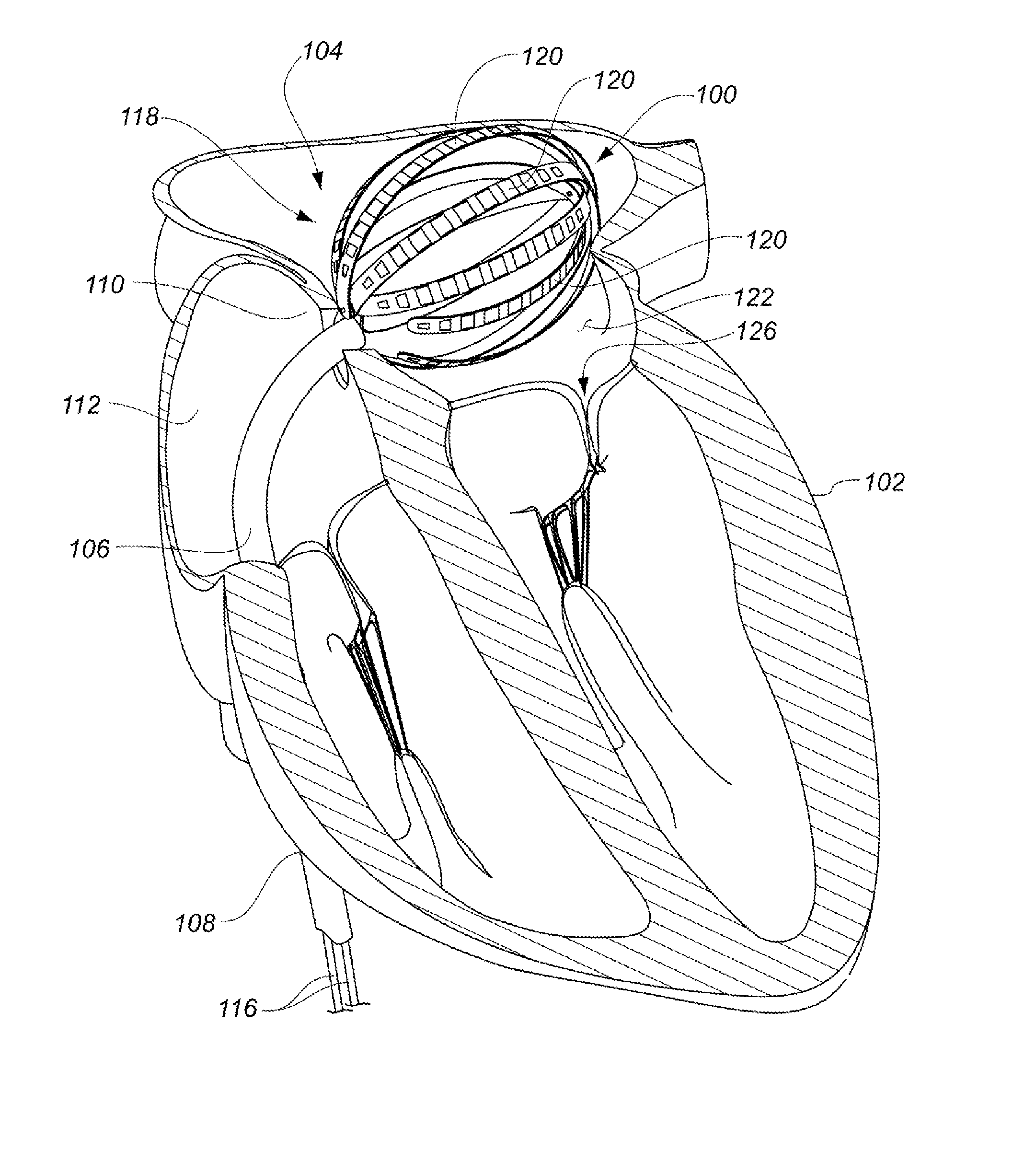
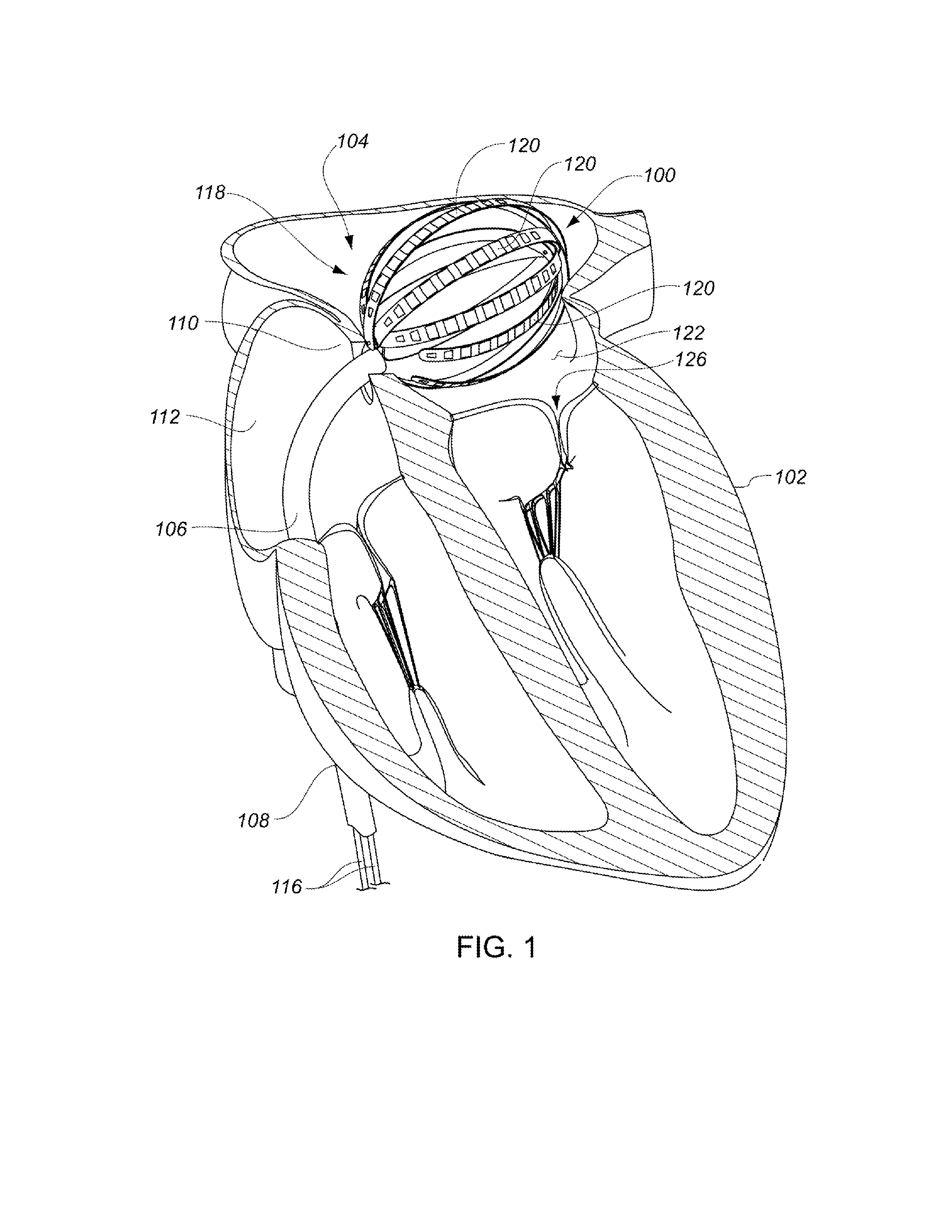
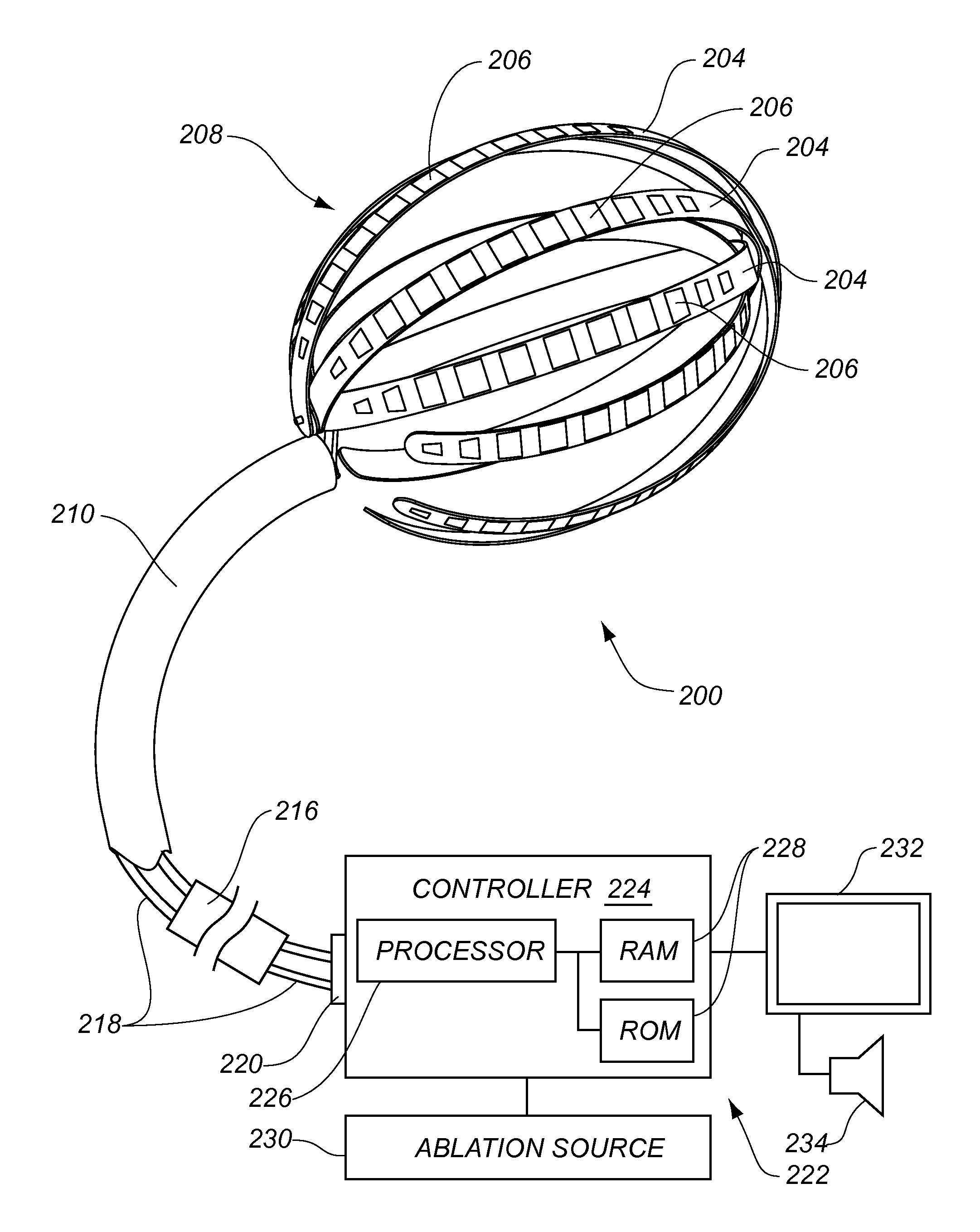
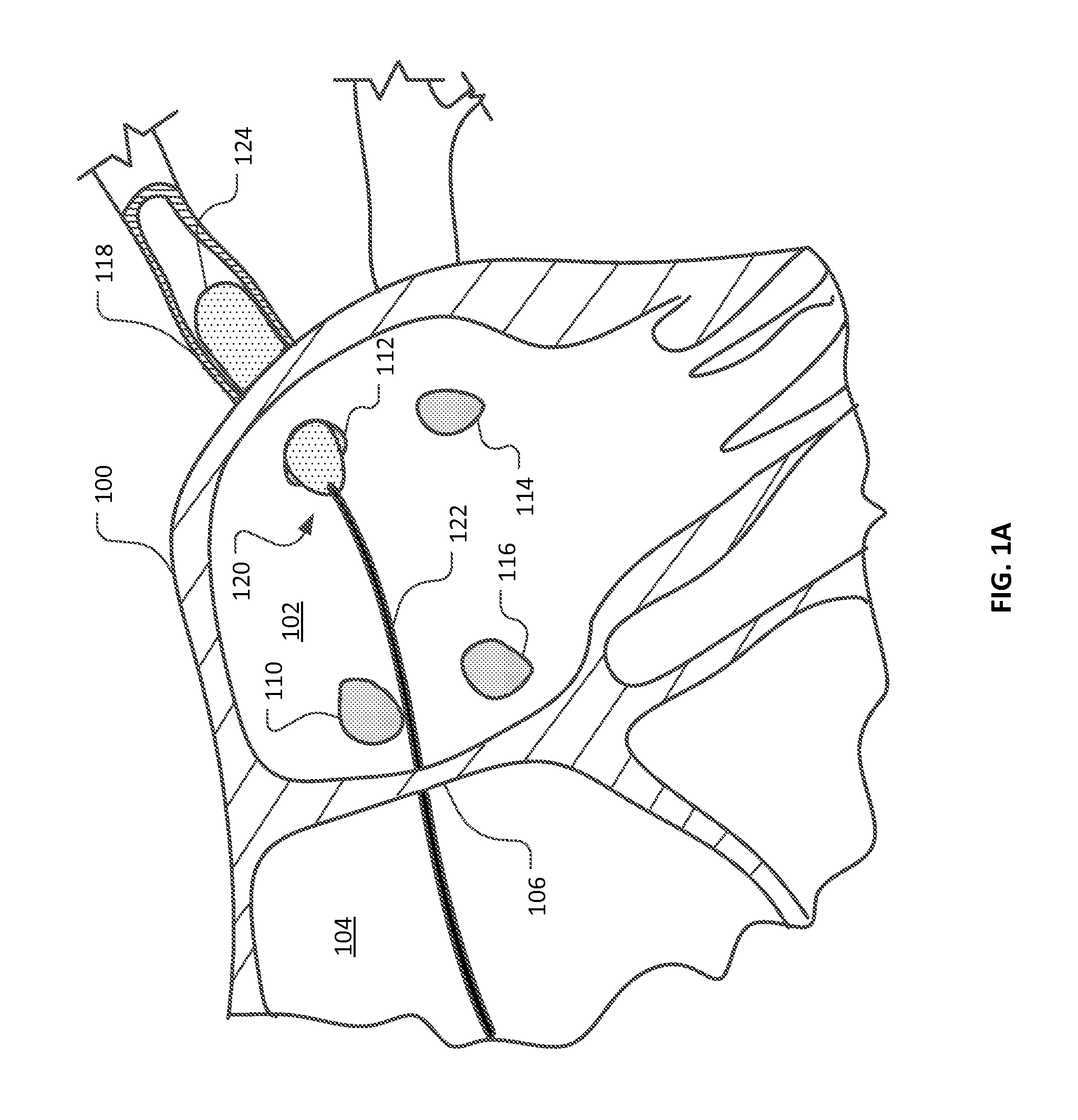
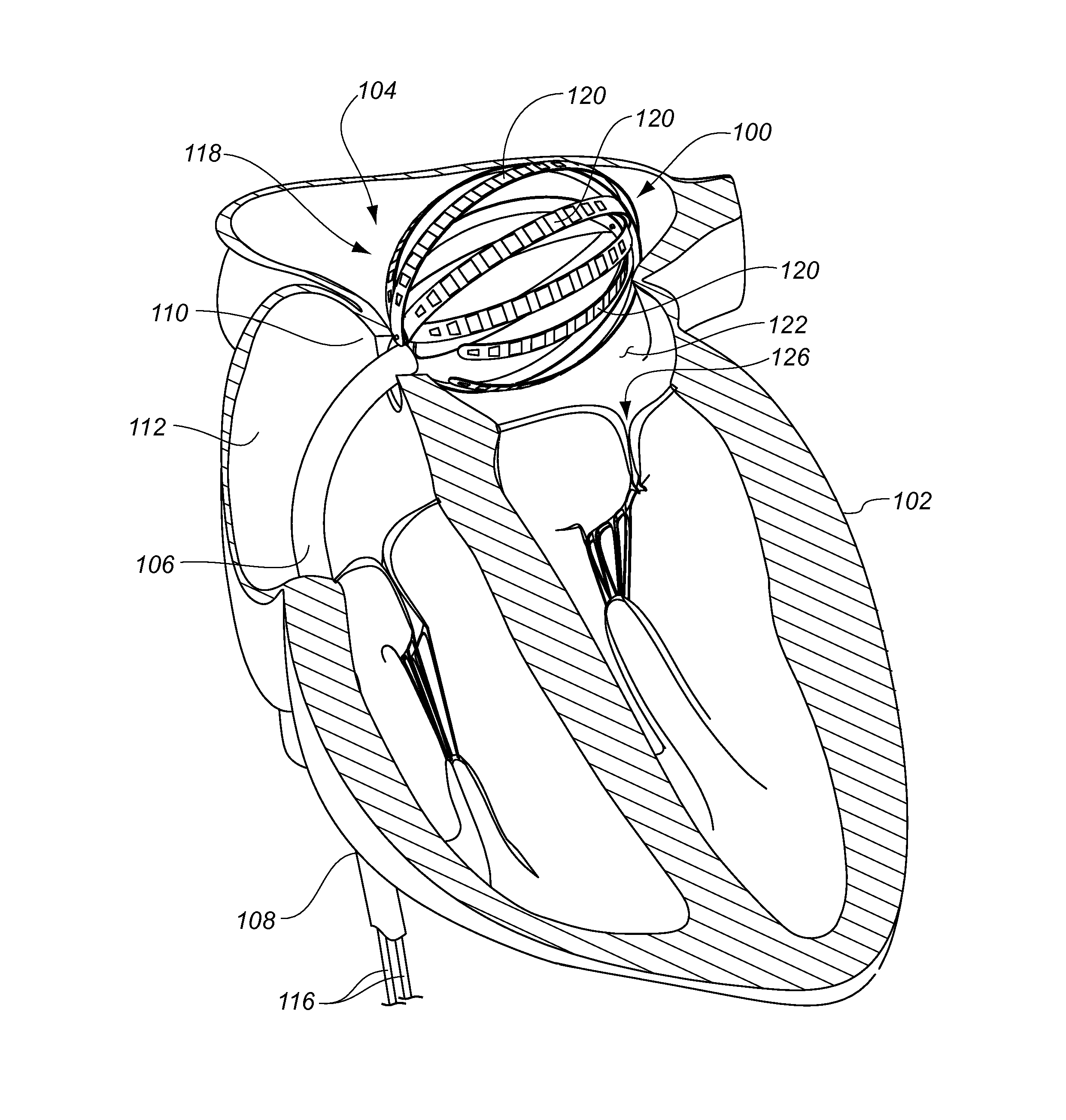
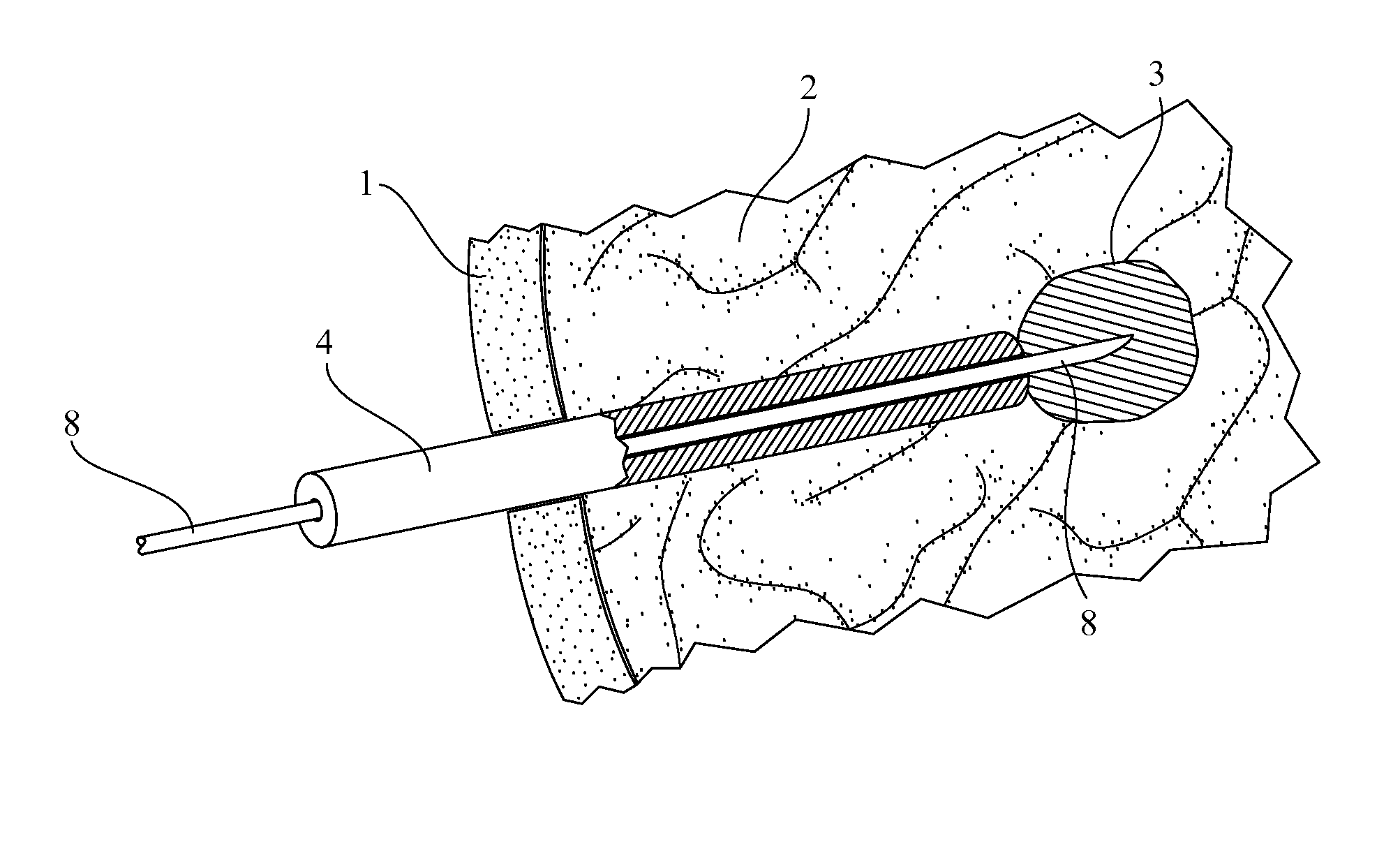
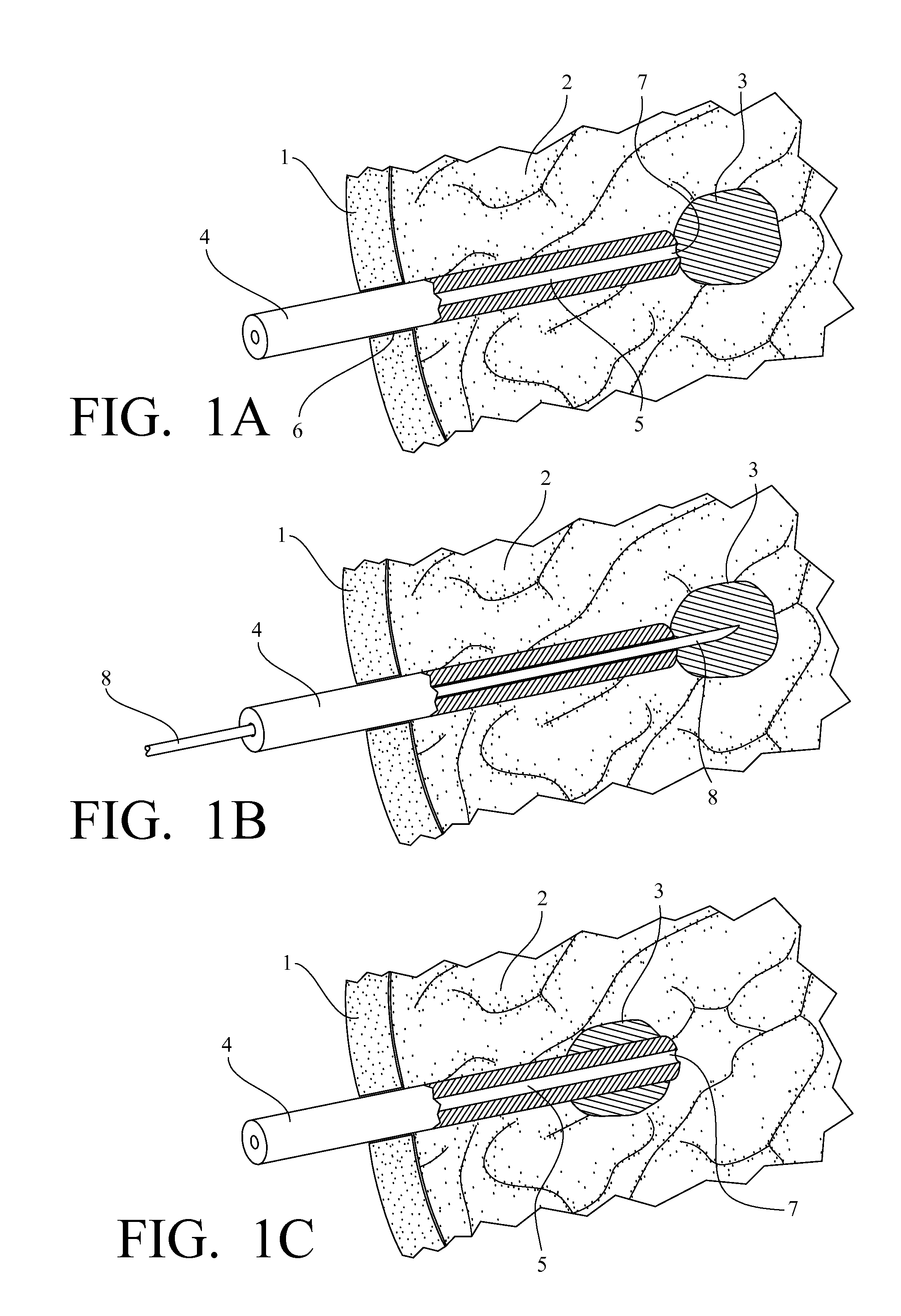
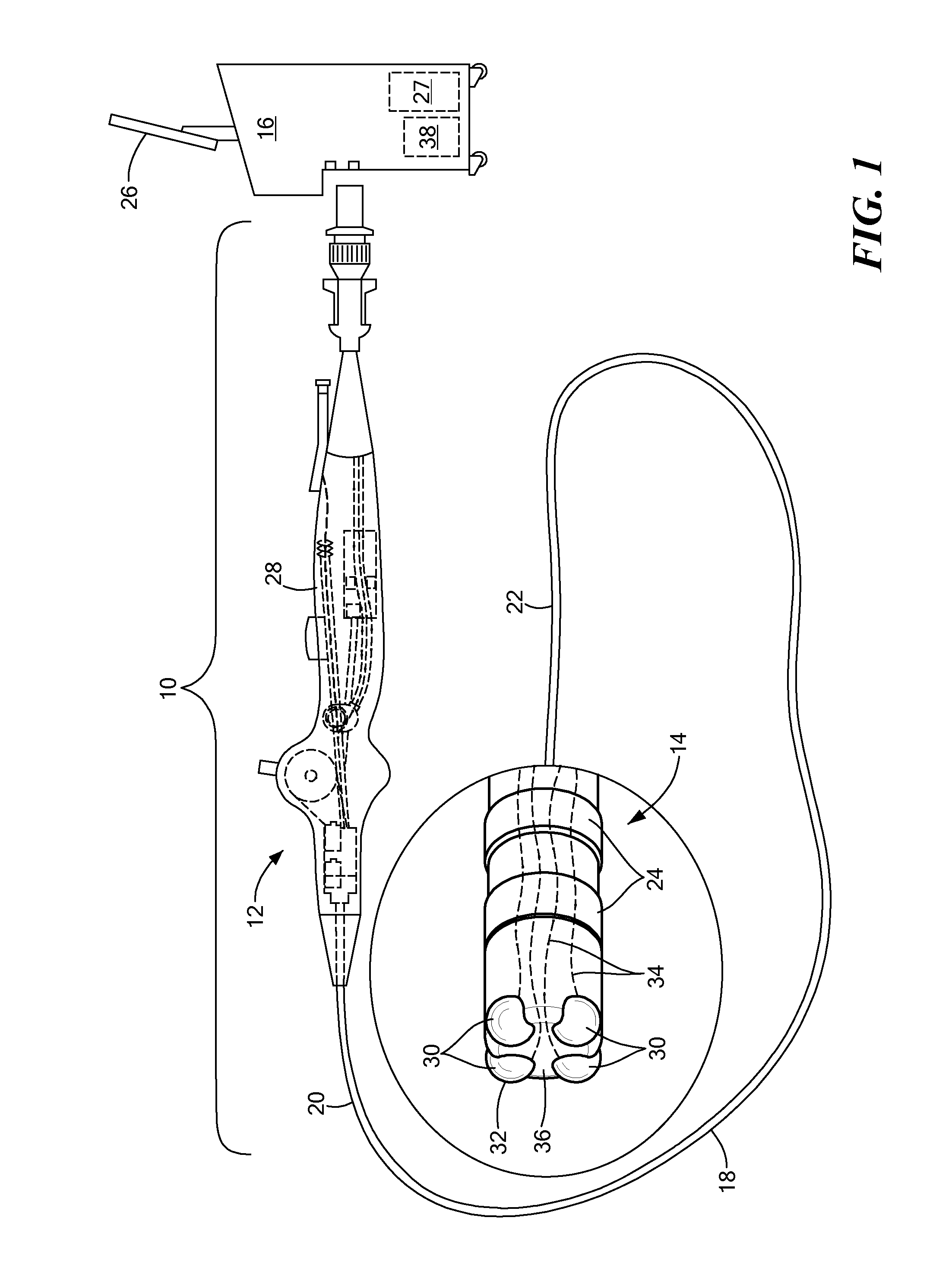
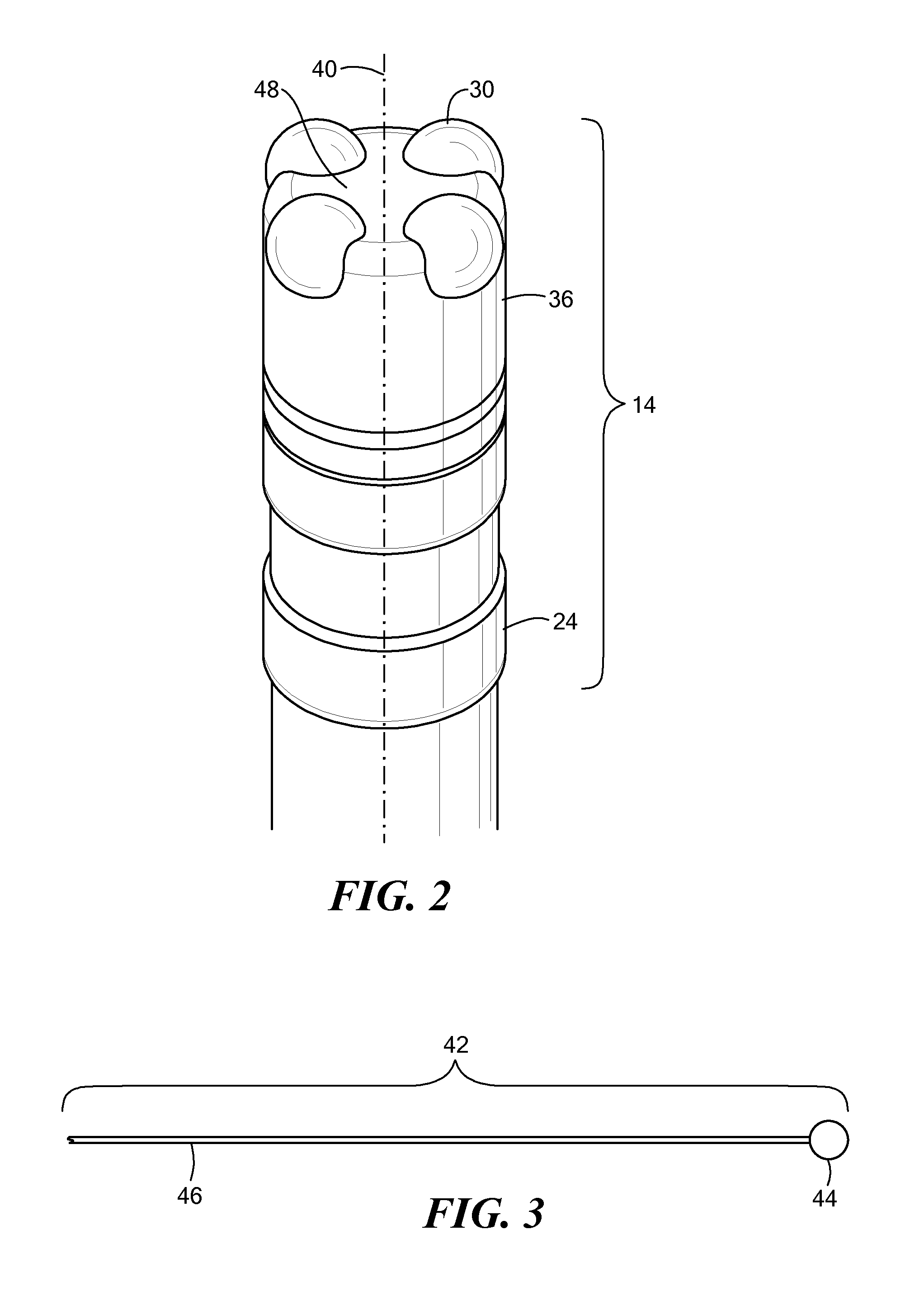
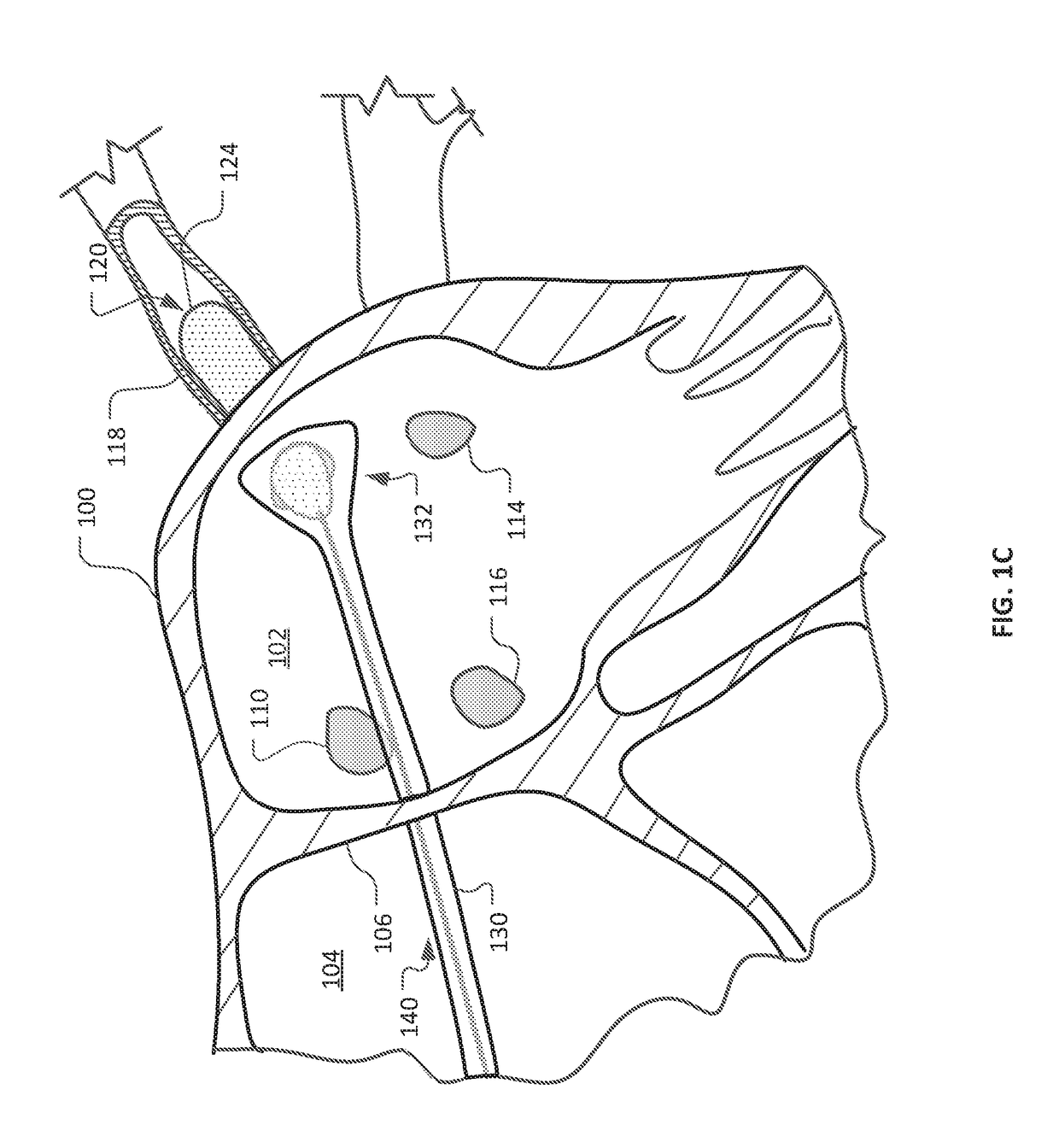
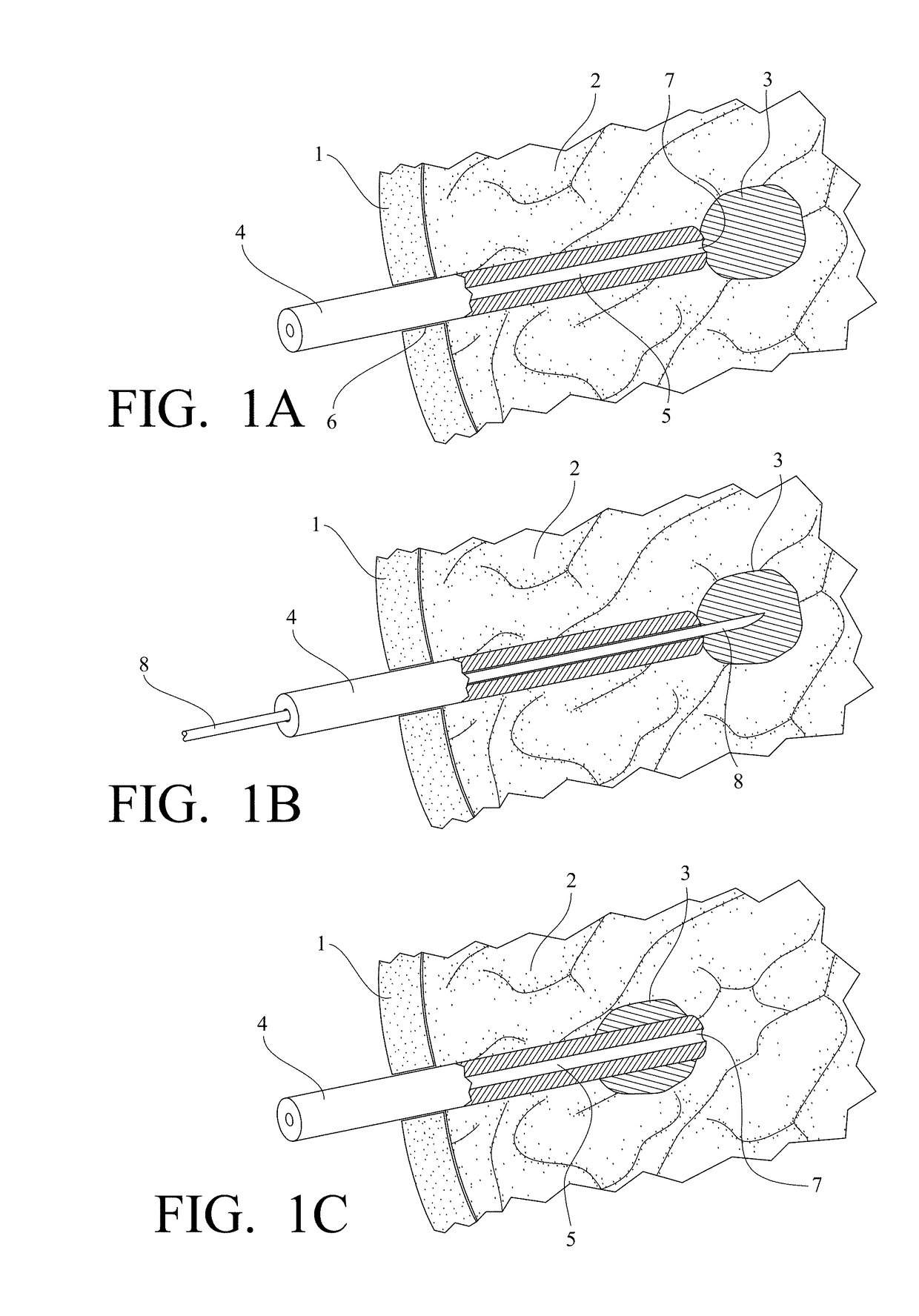
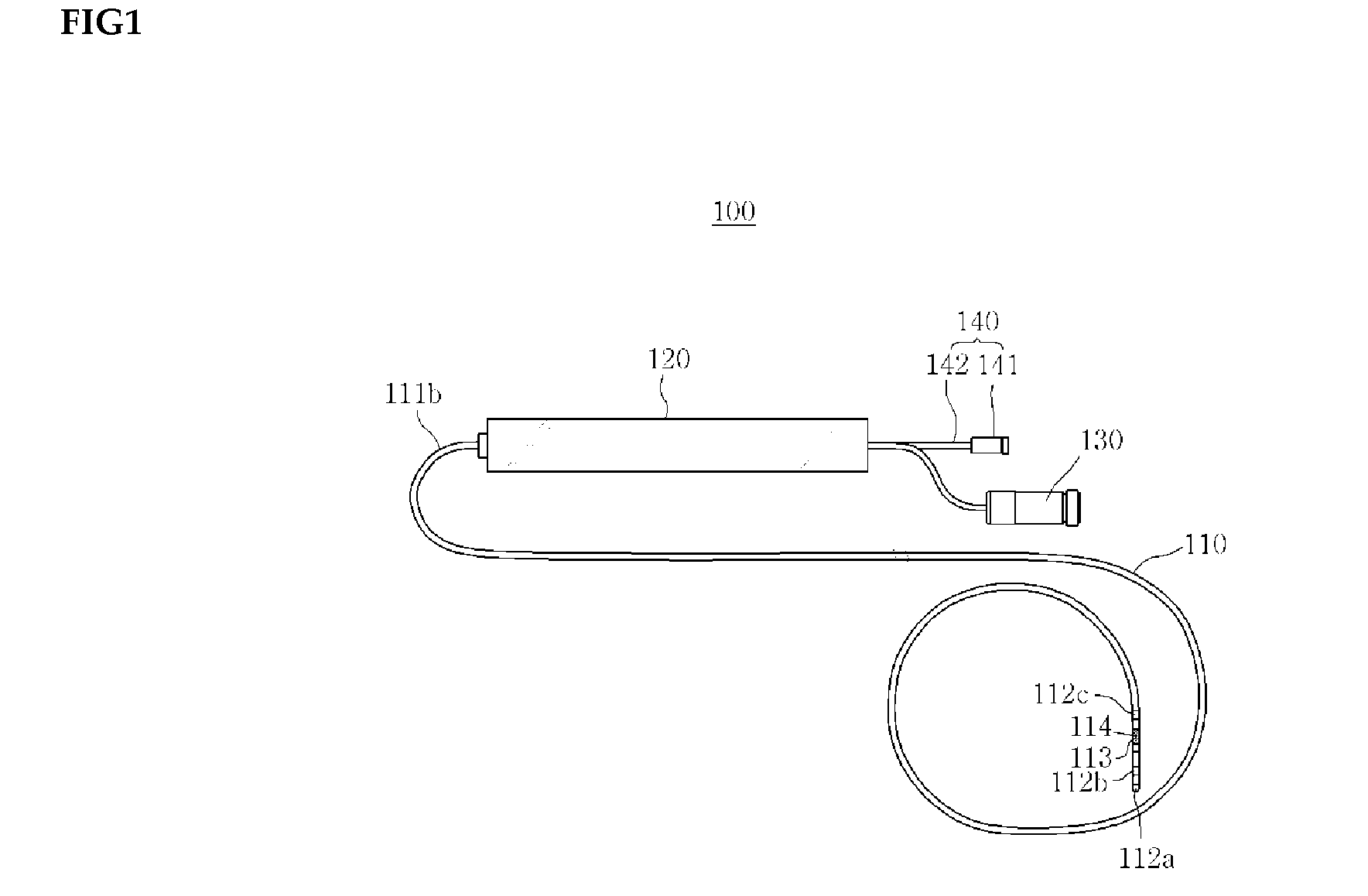
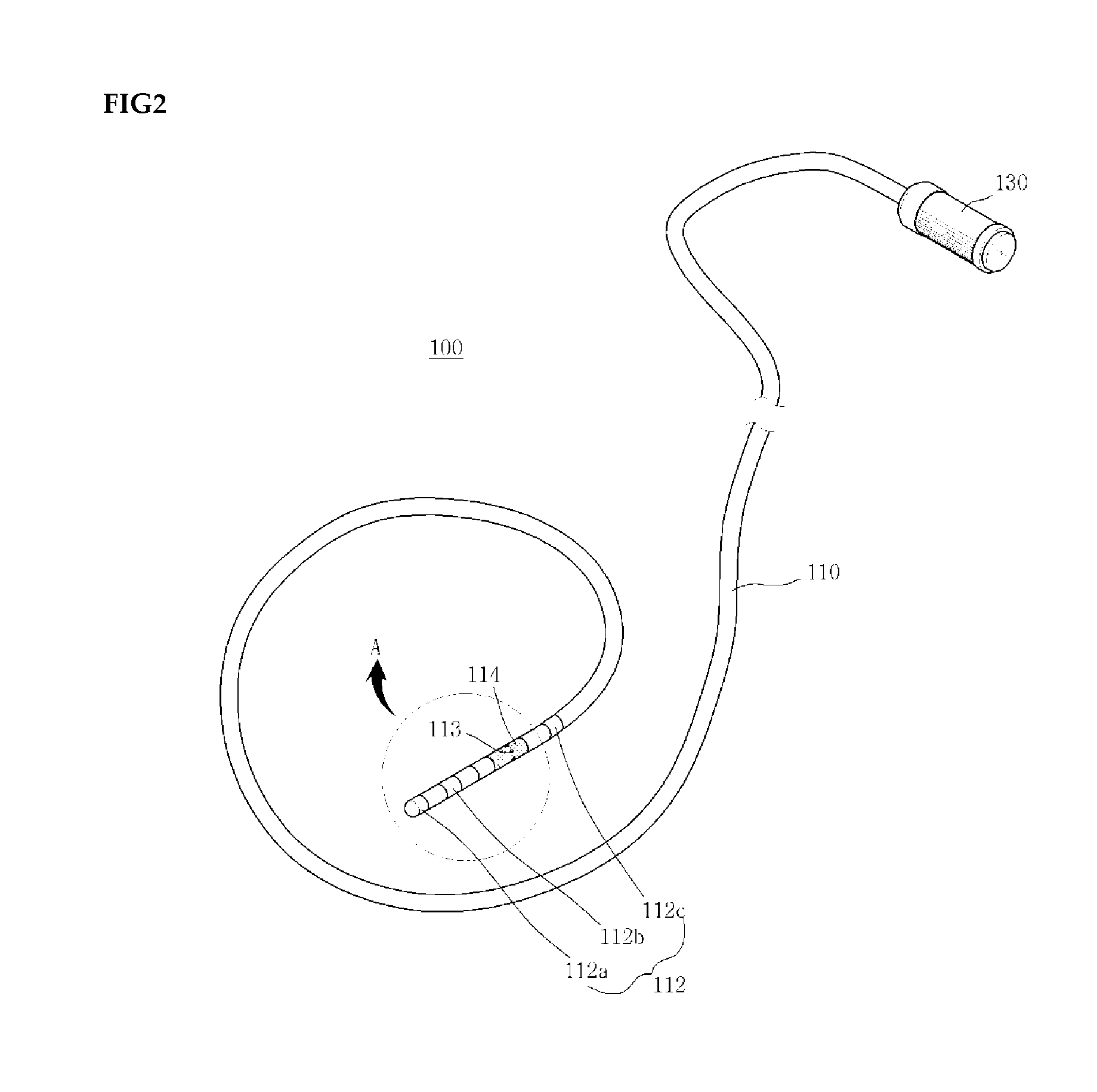
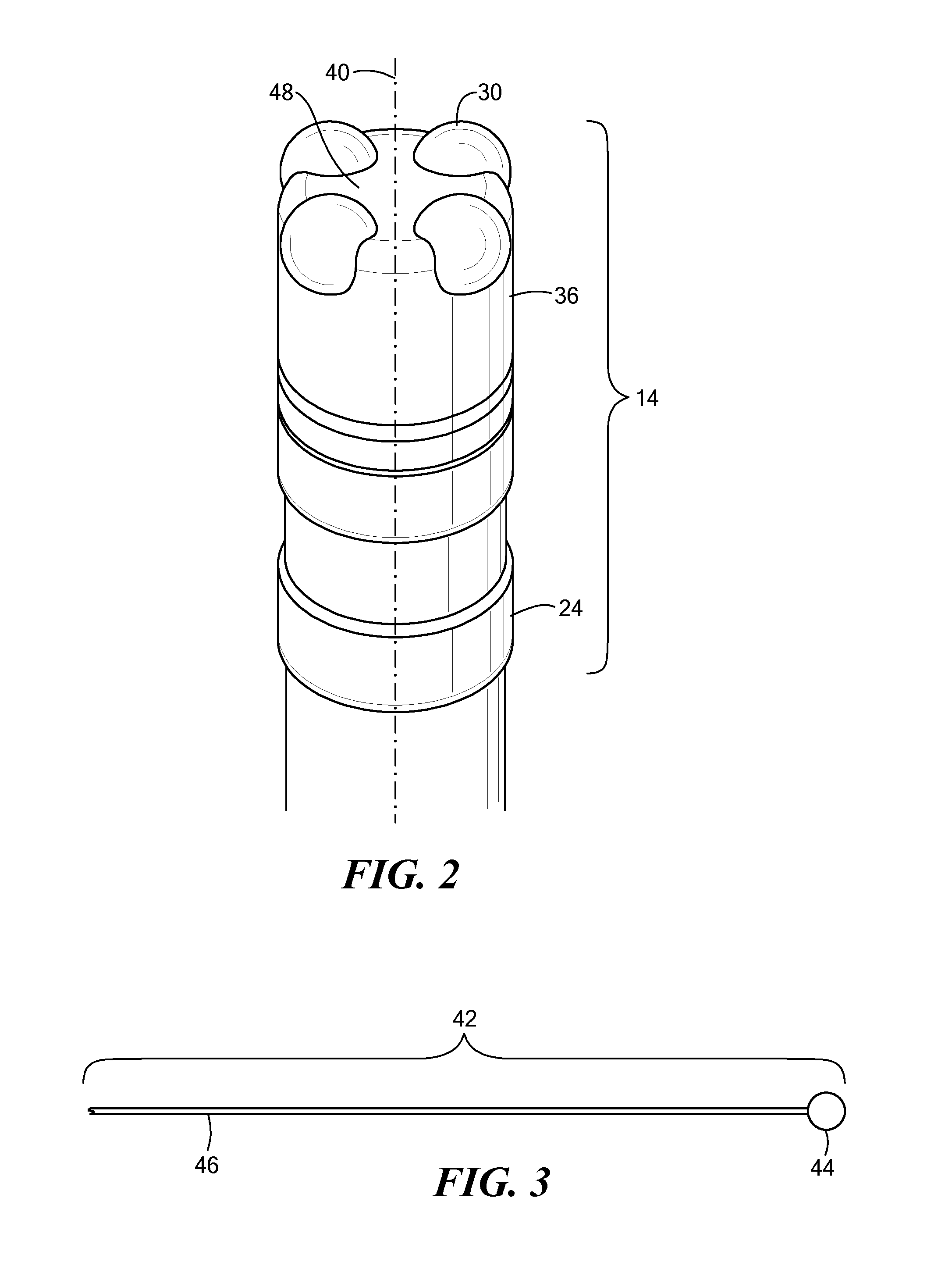
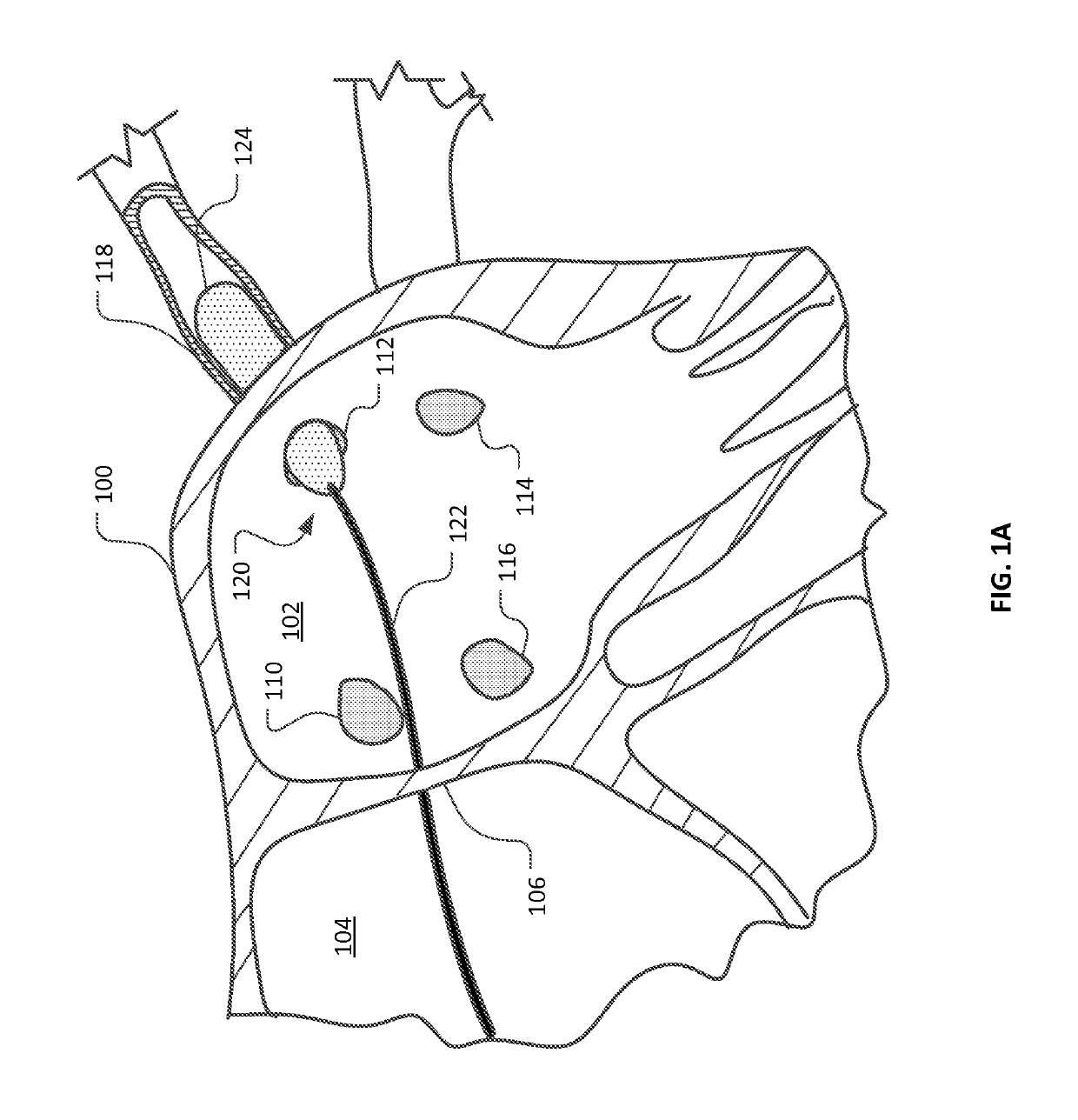
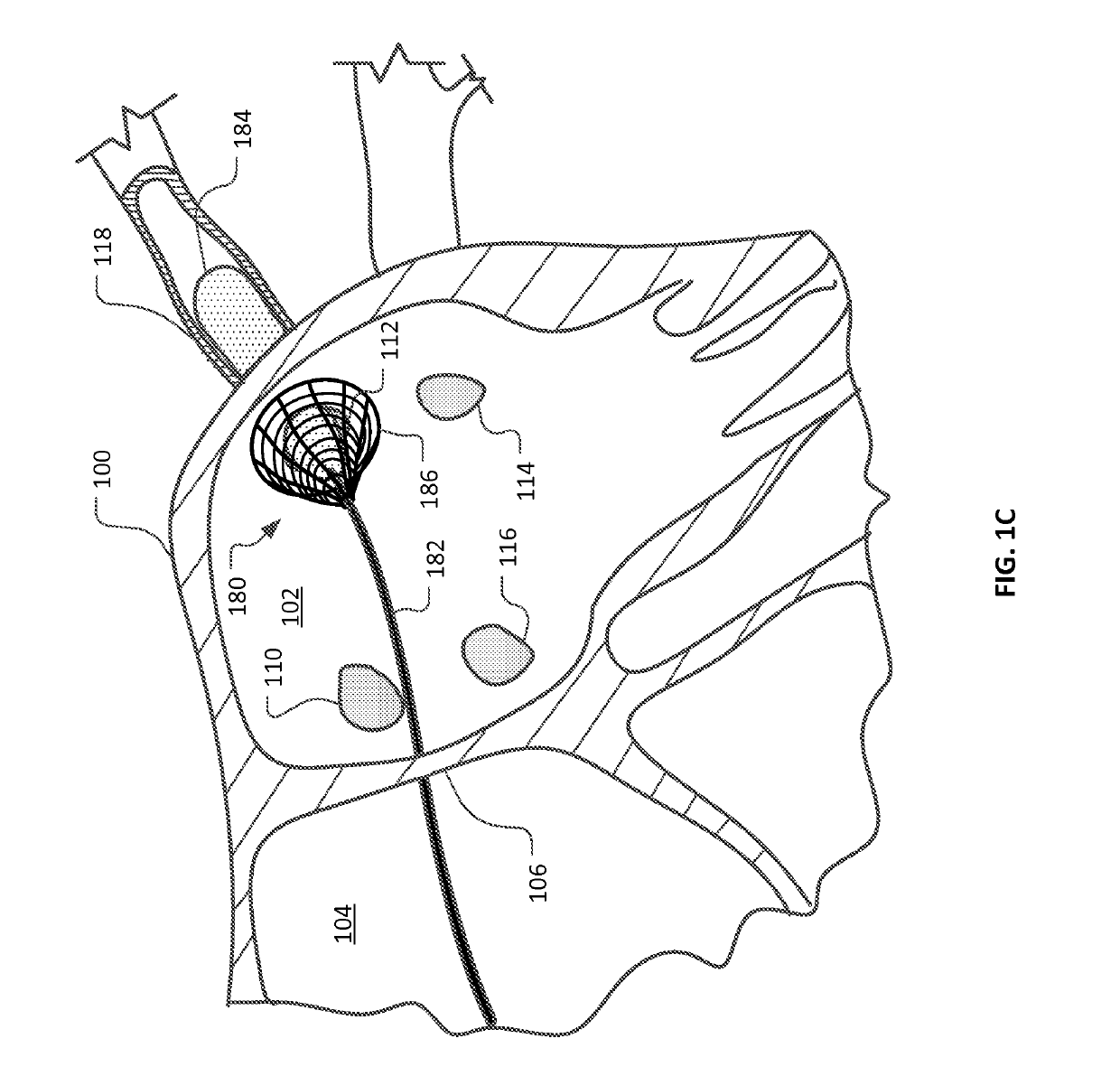
Medical device for use in bodily lumens, for example an atrium
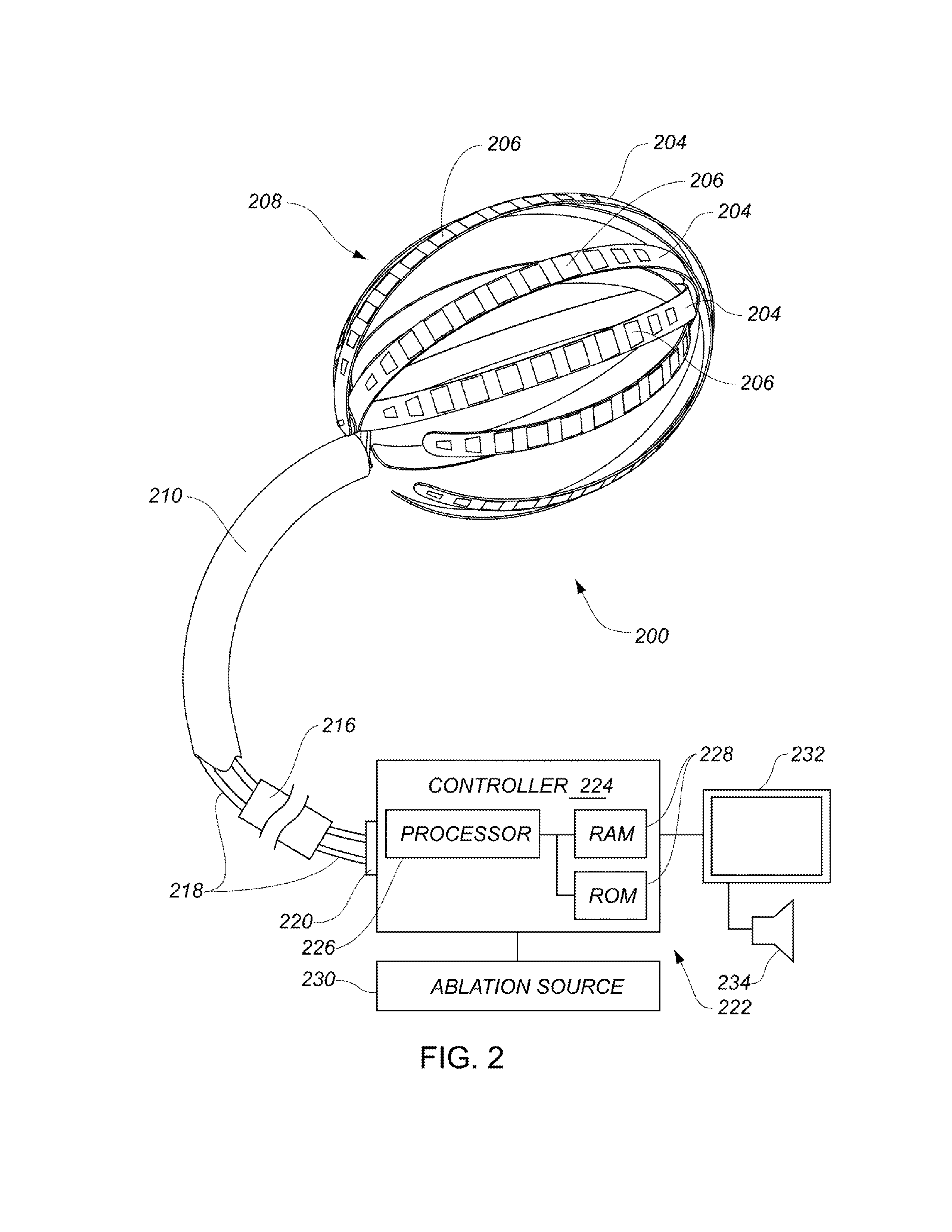
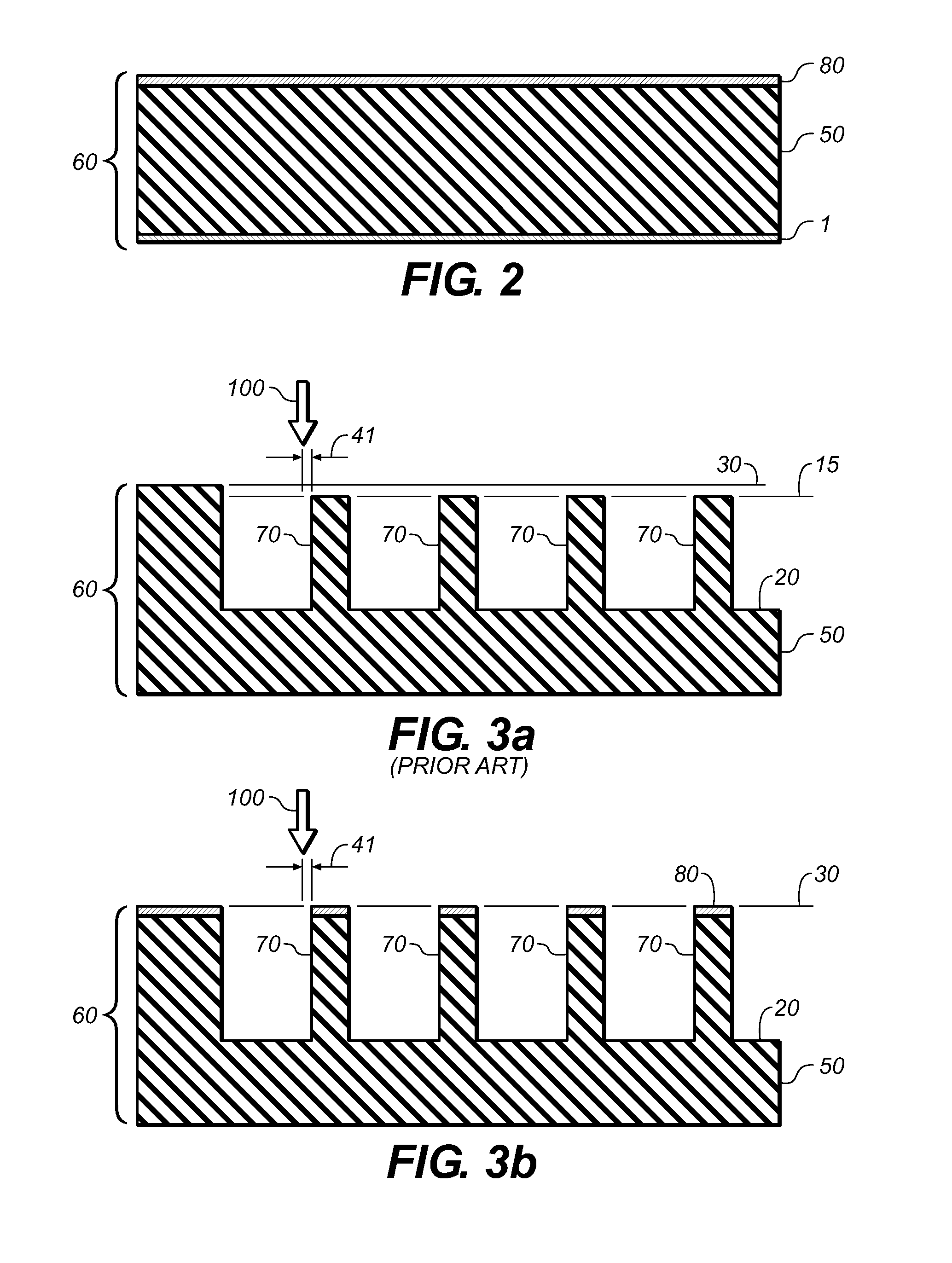
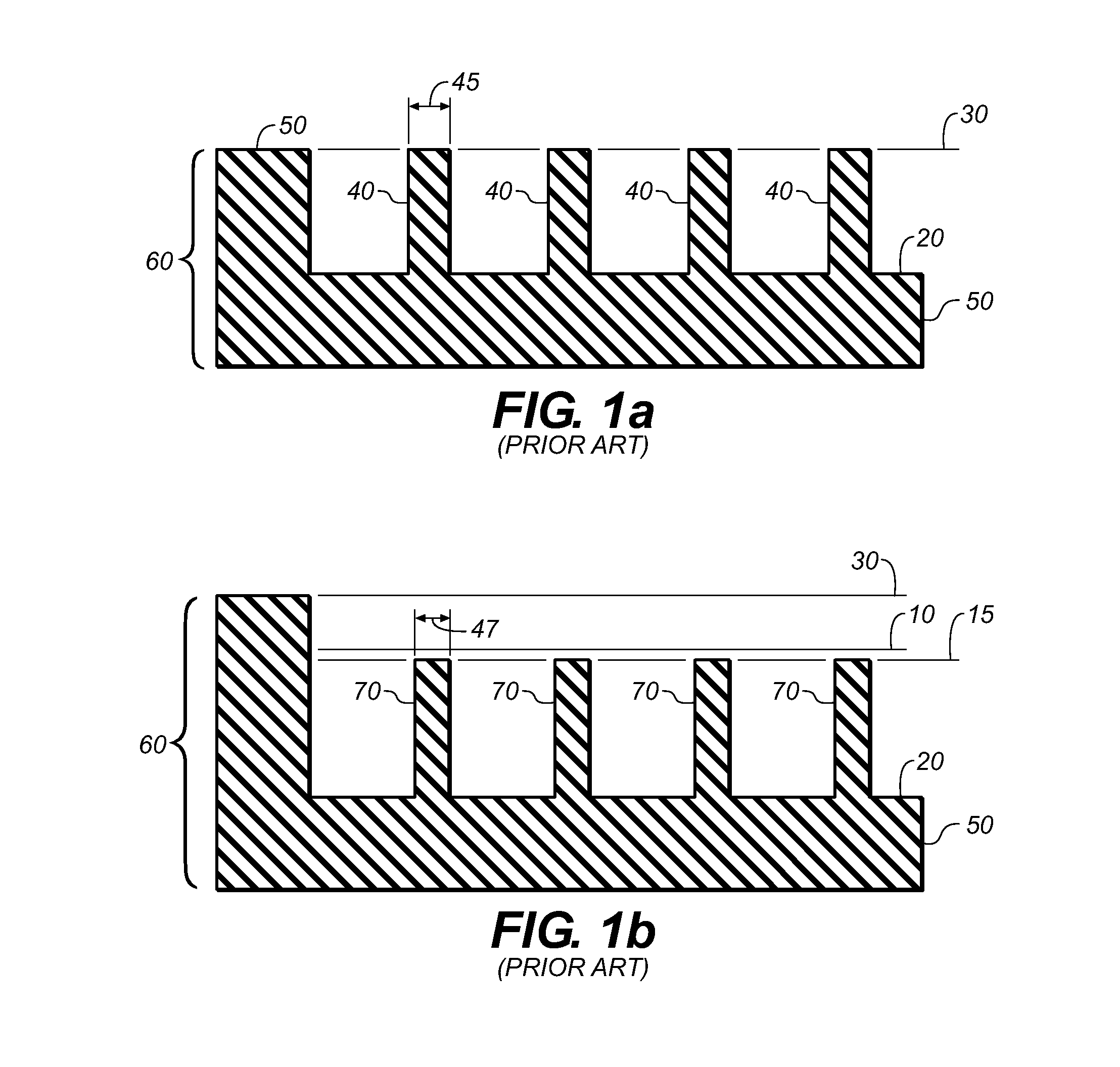
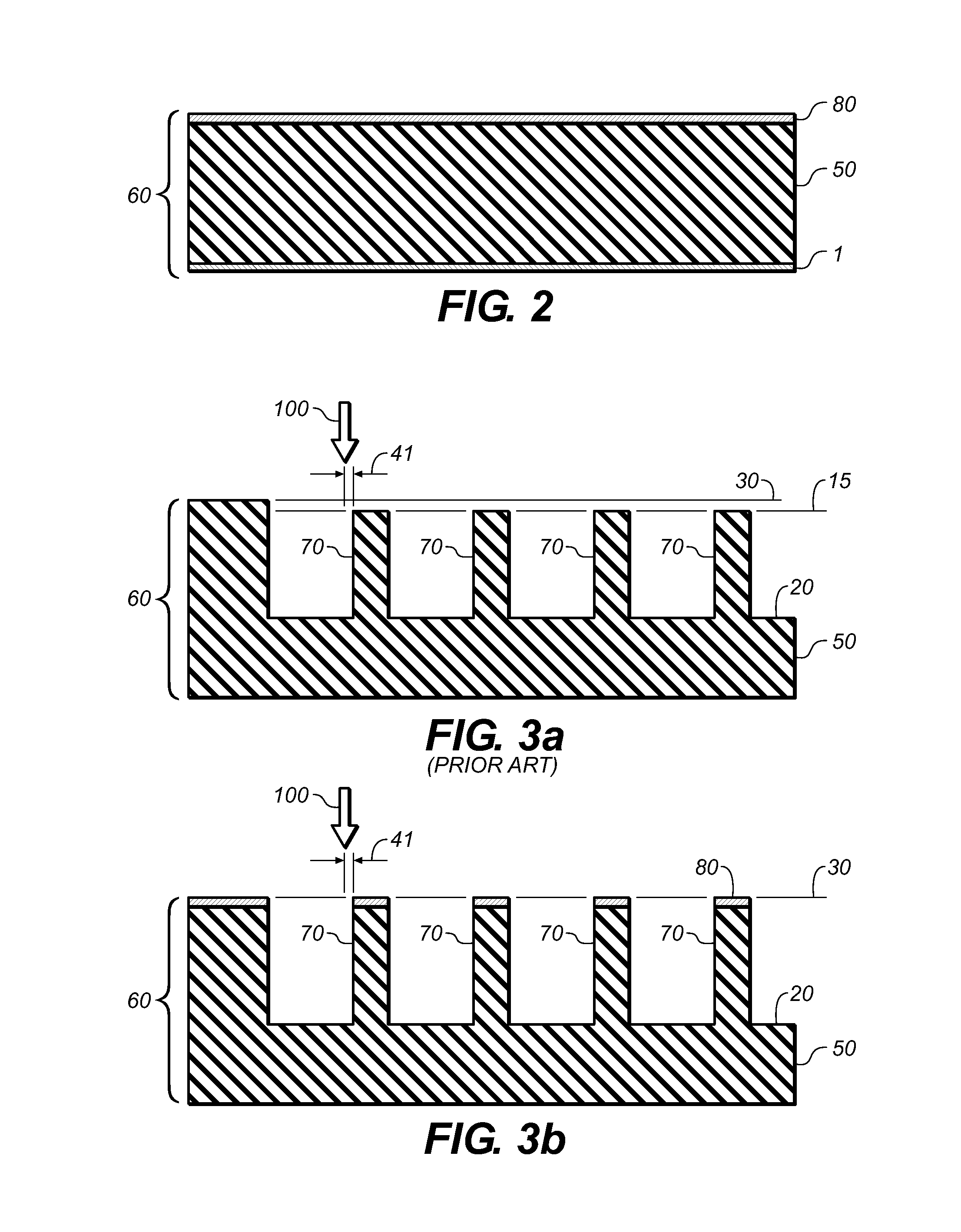
ActiveUS20090131930A1Improve abilitiesConvenient location informationDiagnostic recording/measuringSensorsAtrial cavityPermittivity
A device positionable in a cavity of a bodily organ (e.g., a heart) may discriminate between fluid (e.g., blood) and non-fluid tissue (e.g., wall of heart) to provide information or a mapping indicative of a position and / or orientation of the device in the cavity. Discrimination may be based on flow, or some other characteristic, for example electrical permittivity or force. The device may selectively ablate portions of the non-fluid tissue based on the information or mapping. The device may detect characteristics (e.g., electrical potentials) indicative of whether ablation was successful. The device may include a plurality of transducers, intravascularly guided in an unexpanded configuration and positioned proximate the non-fluid tissue in an expanded configuration. Expansion mechanism may include helical member(s) or inflatable member(s).
Owner:KARDIUM
Medical device for use in bodily lumens, for example an atrium
ActiveUS20110172658A1Increase capacityConvenient location informationSurgical instrument detailsDiagnostic recording/measuringAtrial cavityProximate
A device positionable in a cavity of a bodily organ (e.g., a heart) may discriminate between fluid (e.g., blood) and non-fluid tissue (e.g., wall of heart) to provide information or a mapping indicative of a position and / or orientation of the device in the cavity. Discrimination may be based on flow, or some other characteristic, for example electrical permittivity or force. The device may selectively ablate portions of the non-fluid tissue based on the information or mapping. The device may detect characteristics (e.g., electrical potentials) indicative of whether ablation was successful. The device may include a plurality of transducers, intravascularly guided in an unexpanded configuration and positioned proximate the non-fluid tissue in an expanded configuration. Expansion mechanism may include helical member(s) or inflatable member(s).
Owner:KARDIUM
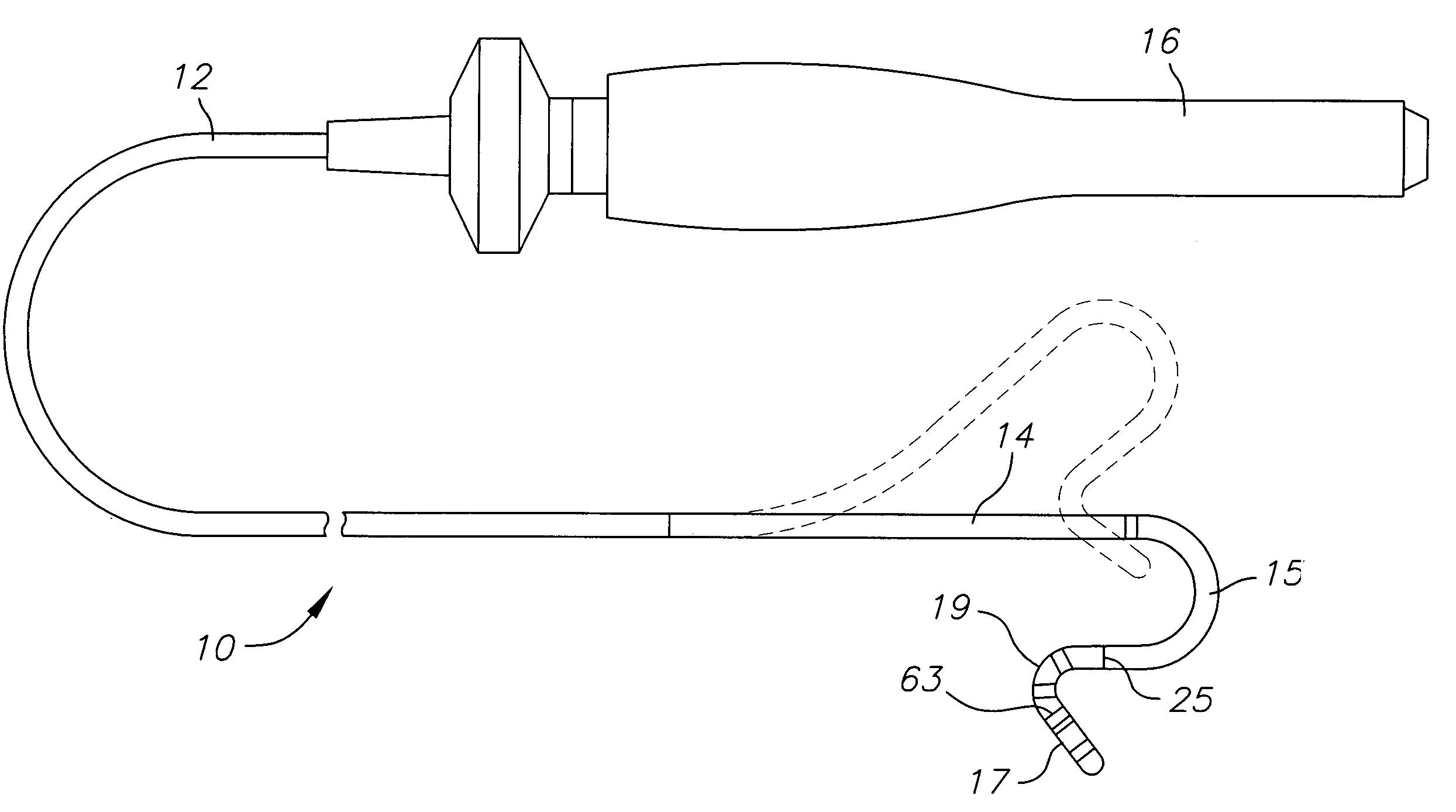
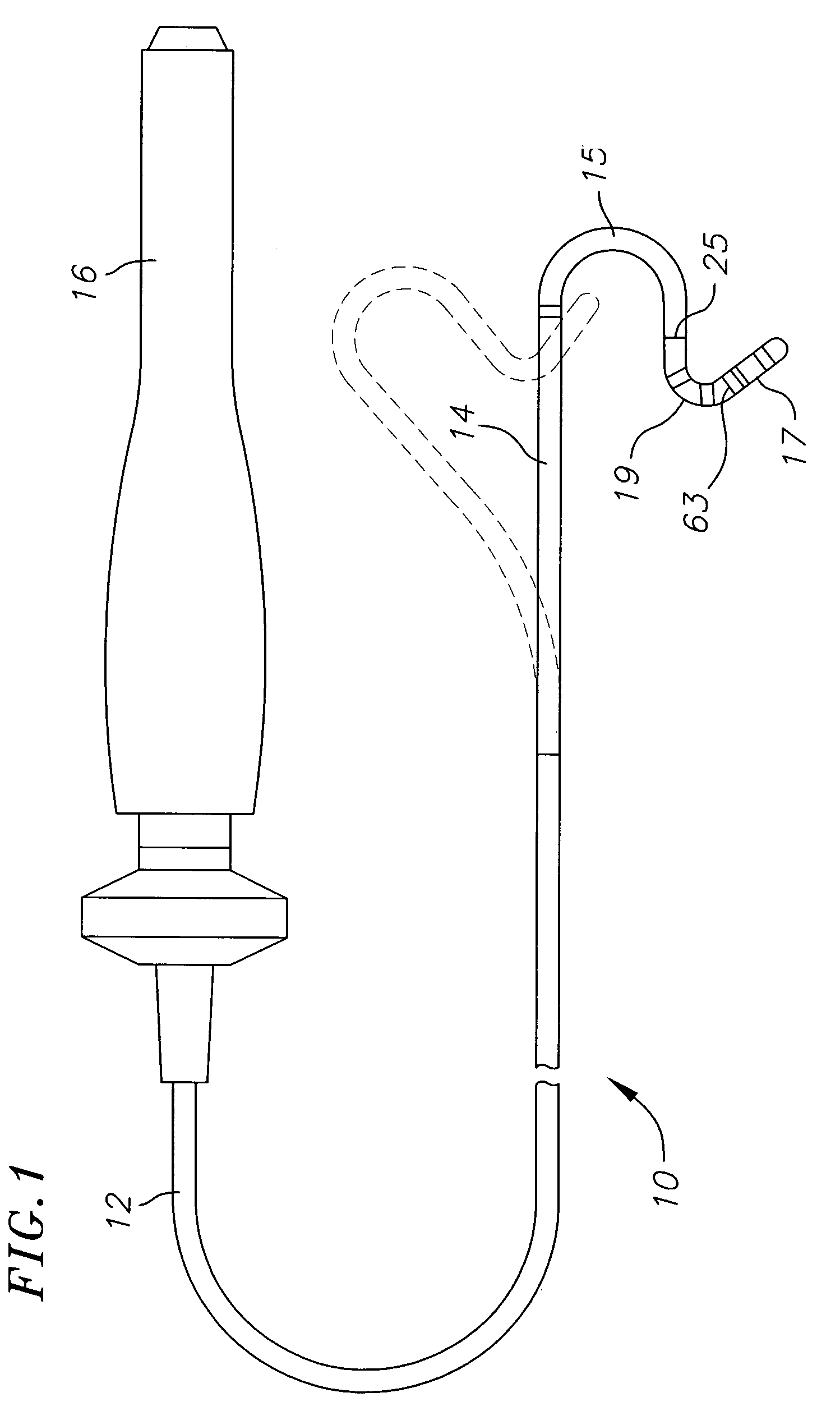
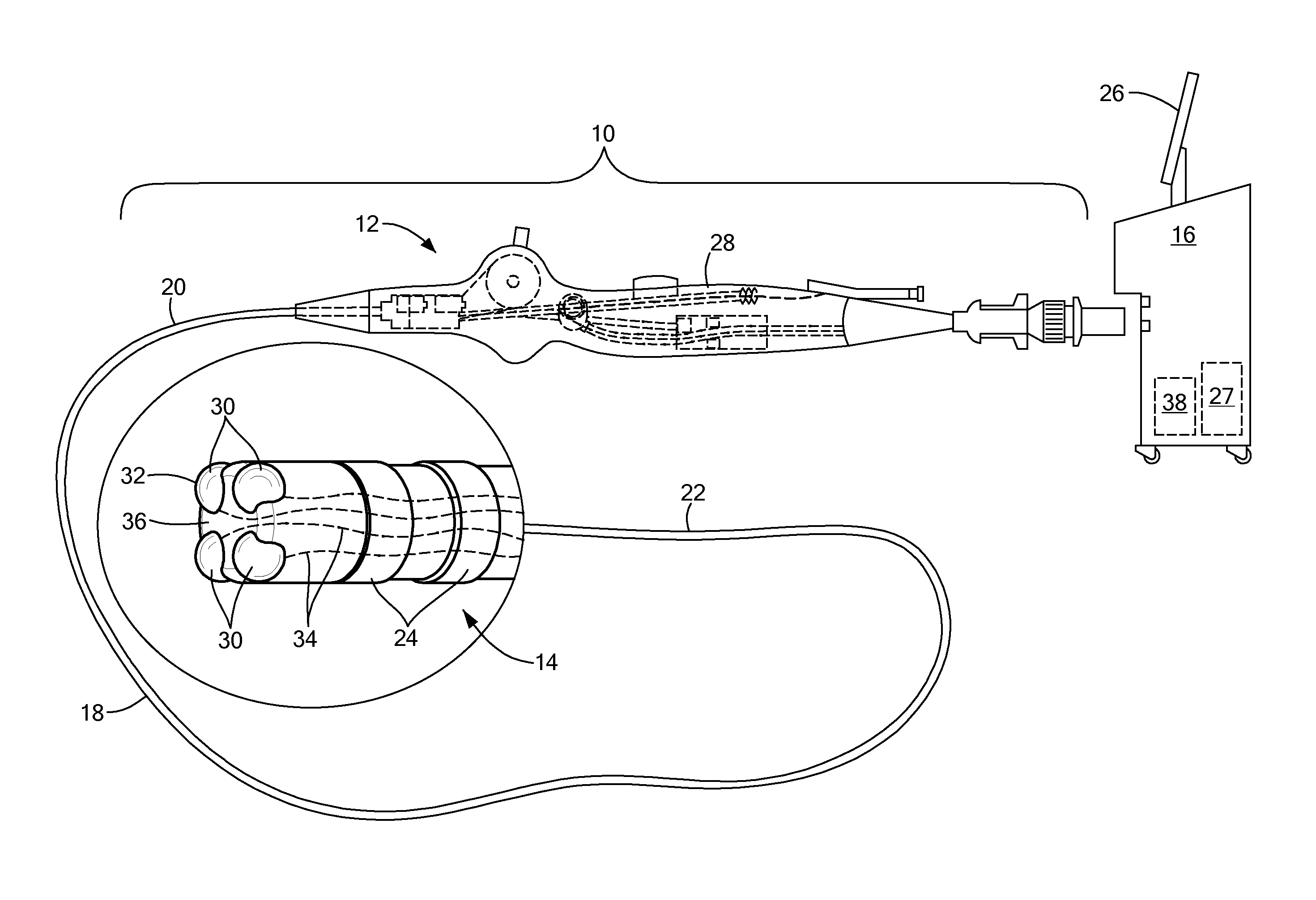
Catheter with flexible pre-shaped tip section
ActiveUS7623899B2Improve security featuresImprove abilitiesMulti-lumen catheterDiagnostic recording/measuringVeinLeft atrium
A catheter for mapping and / or ablating continuous linear or circumferential lesions at the intersection of a generally flat structure, such as the left atrium, and the ostium of generally cavernous regions of the heart, including pulmonary vein and the pulmonary venous antrum, comprises a catheter body with an intermediate section that is connected to a tip assembly by a highly flexible section. The intermediate section has at its distal end a preformed section, e.g., a curve, the intermediate section being deflectable in a direction opposite to the curve. The highly flexible section presets the tip assembly at an off-axis and / or off-plane angles from the preformed section. Accordingly, the preformed section is adapted to sit in the region and the preset angles of the ablation assembly enable contact with surrounding tissue. A high bending modulus enables the flexible section absorb displacement force applied to the ablation assembly, such as when the tip assembly encounters uneven tissue surface, without displacing the curve from the region. The tip assembly can be irrigated as enabled by a plurality of irrigation ports, a coil electrode, and a porous covering to disperse fluid over the outer surface of the tip assembly.
Owner:BIOSENSE WEBSTER INC
Catheter with flexible pre-shaped tip section
ActiveUS20070066878A1Improve abilityImprove safety featureMulti-lumen catheterDiagnostic recording/measuringWater irrigationCorneal ablation
A catheter for mapping and / or ablating continuous linear or circumferential lesions at the intersection of a generally flat structure, such as the left atrium, and the ostium of generally cavernous regions of the heart, including pulmonary vein and the pulmonary venous antrum, comprises a catheter body with an intermediate section that is connected to a tip assembly by a highly flexible section. The intermediate section has at its distal end a preformed section, e.g., a curve, the intermediate section being deflectable in a direction opposite to the curve. The highly flexible section presets the tip assembly at an off-axis and / or off-plane angles from the preformed section. Accordingly, the preformed section is adapted to sit in the region and the preset angles of the ablation assembly enable contact with surrounding tissue. A high bending modulus enables the flexible section absorb displacement force applied to the ablation assembly, such as when the tip assembly encounters uneven tissue surface, without displacing the curve from the region. The tip assembly can be irrigated as enabled by a plurality of irrigation ports, a coil electrode, and a porous covering to disperse fluid over the outer surface of the tip assembly.
Owner:BIOSENSE WEBSTER INC
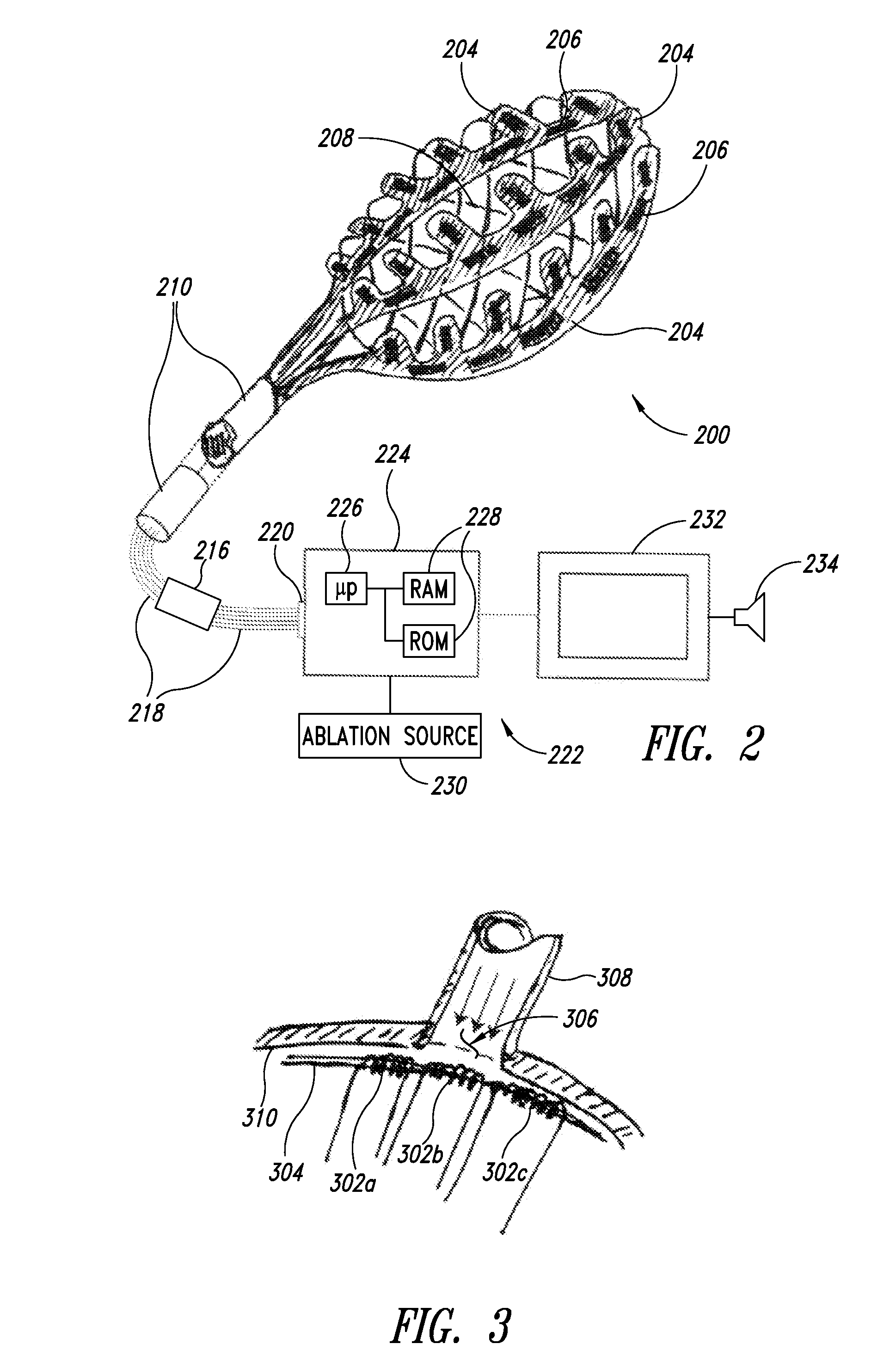
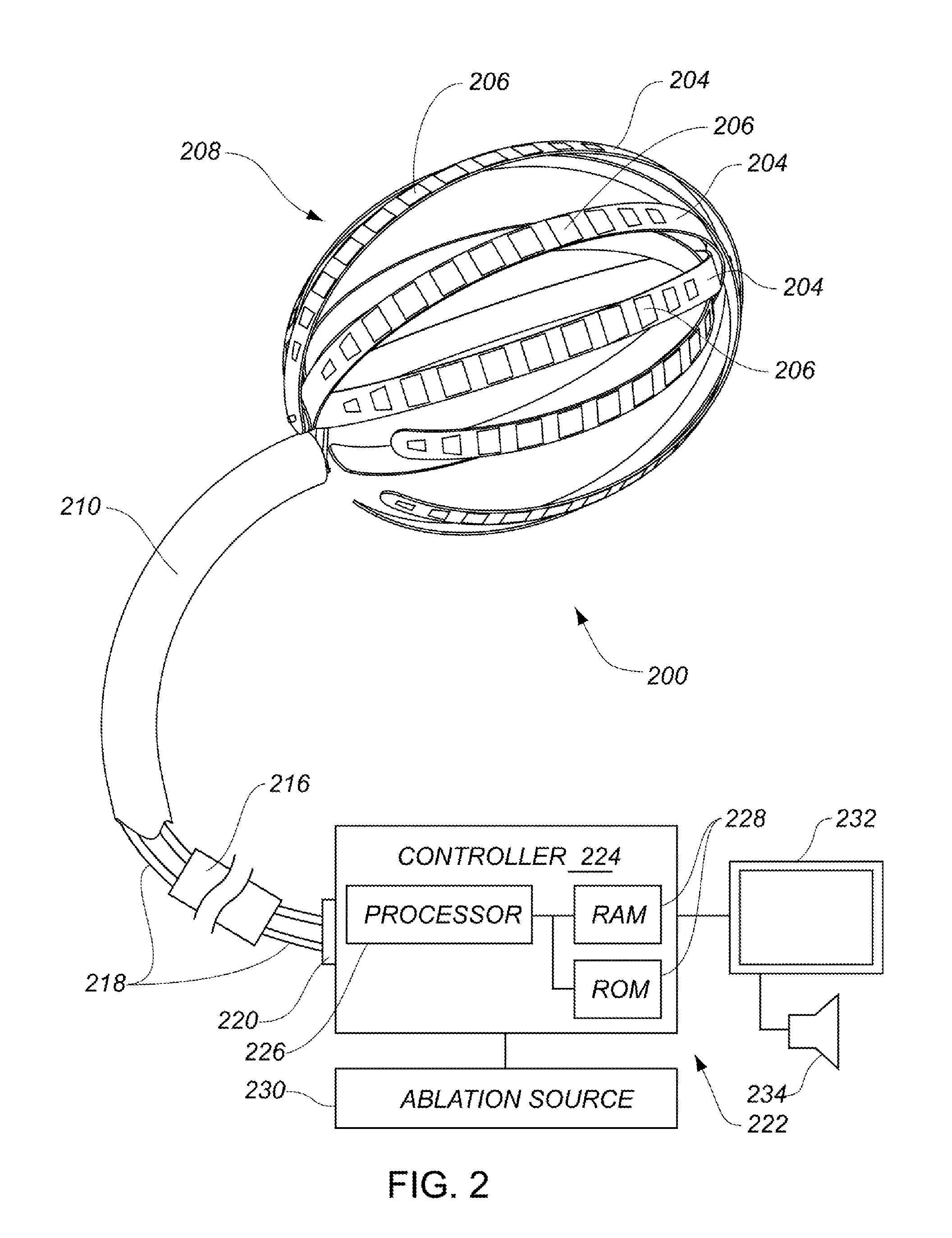
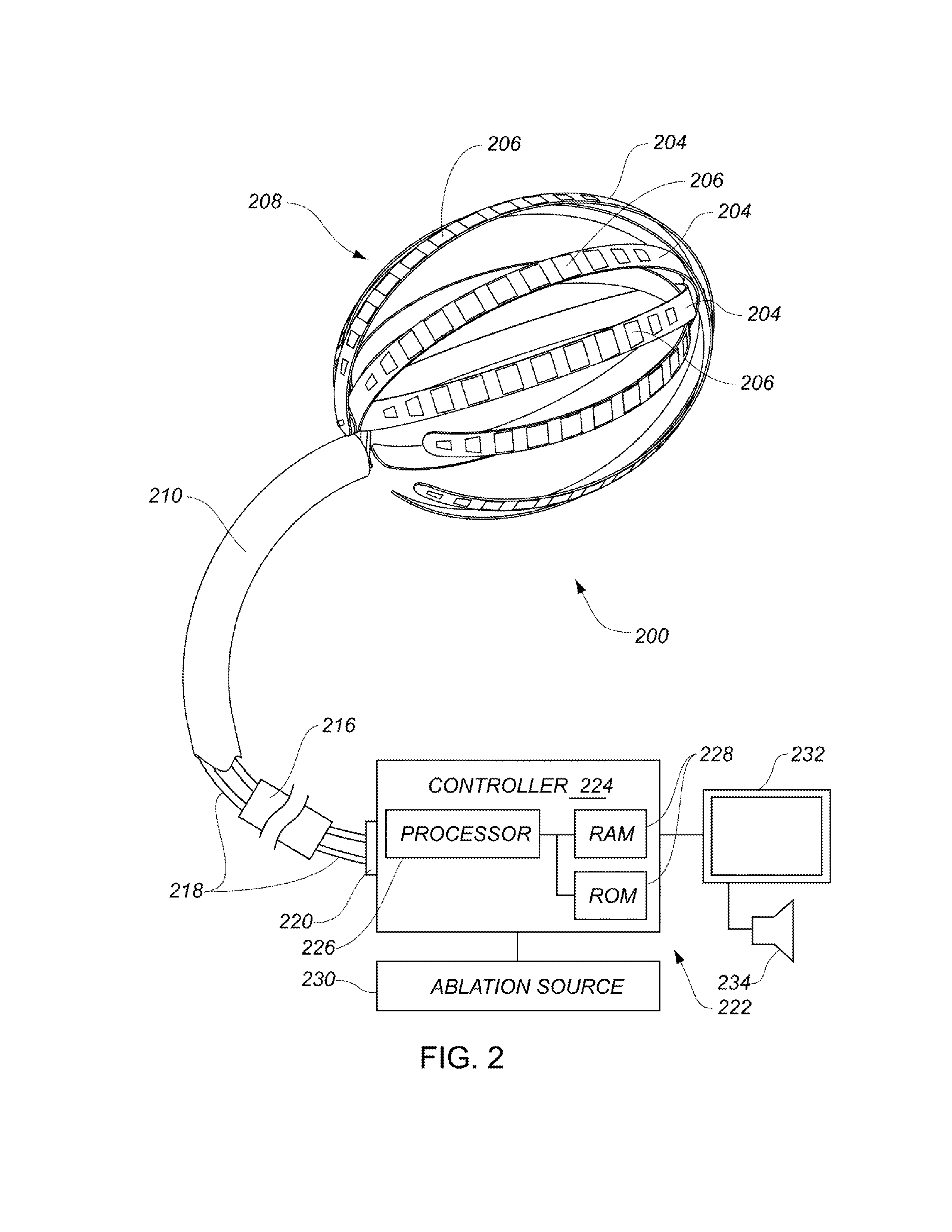
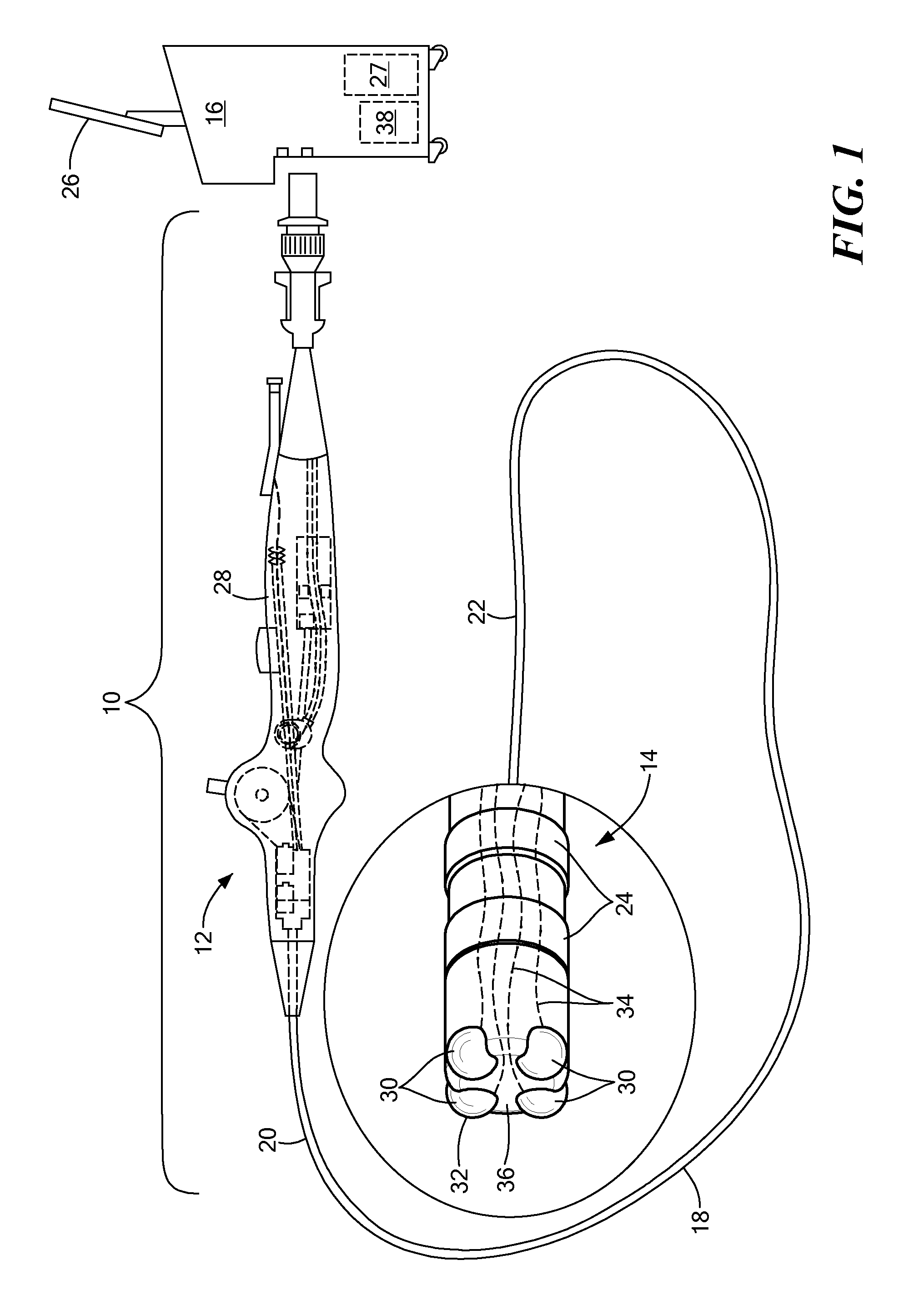
Enhanced medical device for use in bodily cavities, for example an atrium
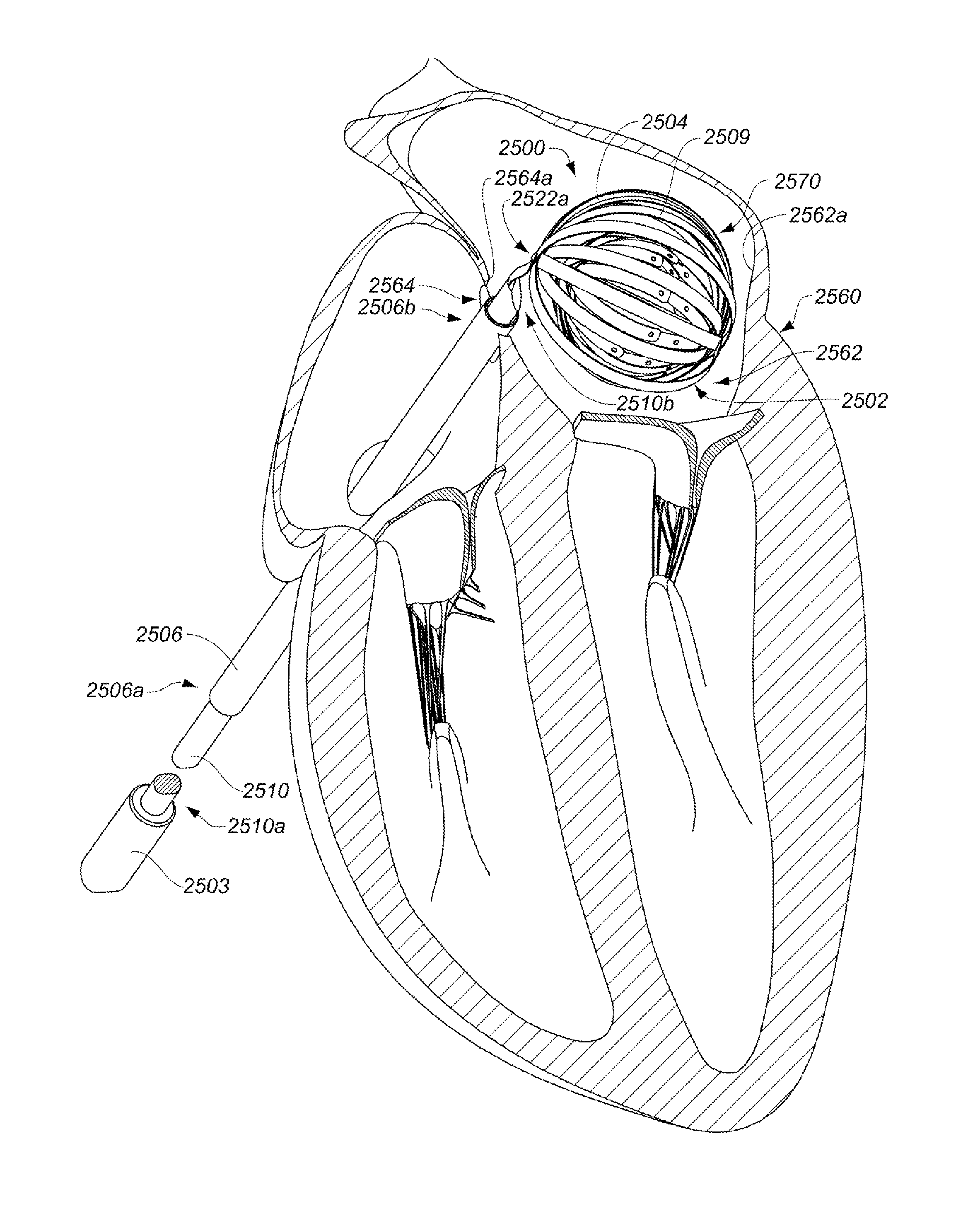
ActiveUS20130304065A1Enhanced for deployment and positioningImprove ablationCatheterSensorsTransducerMedical device
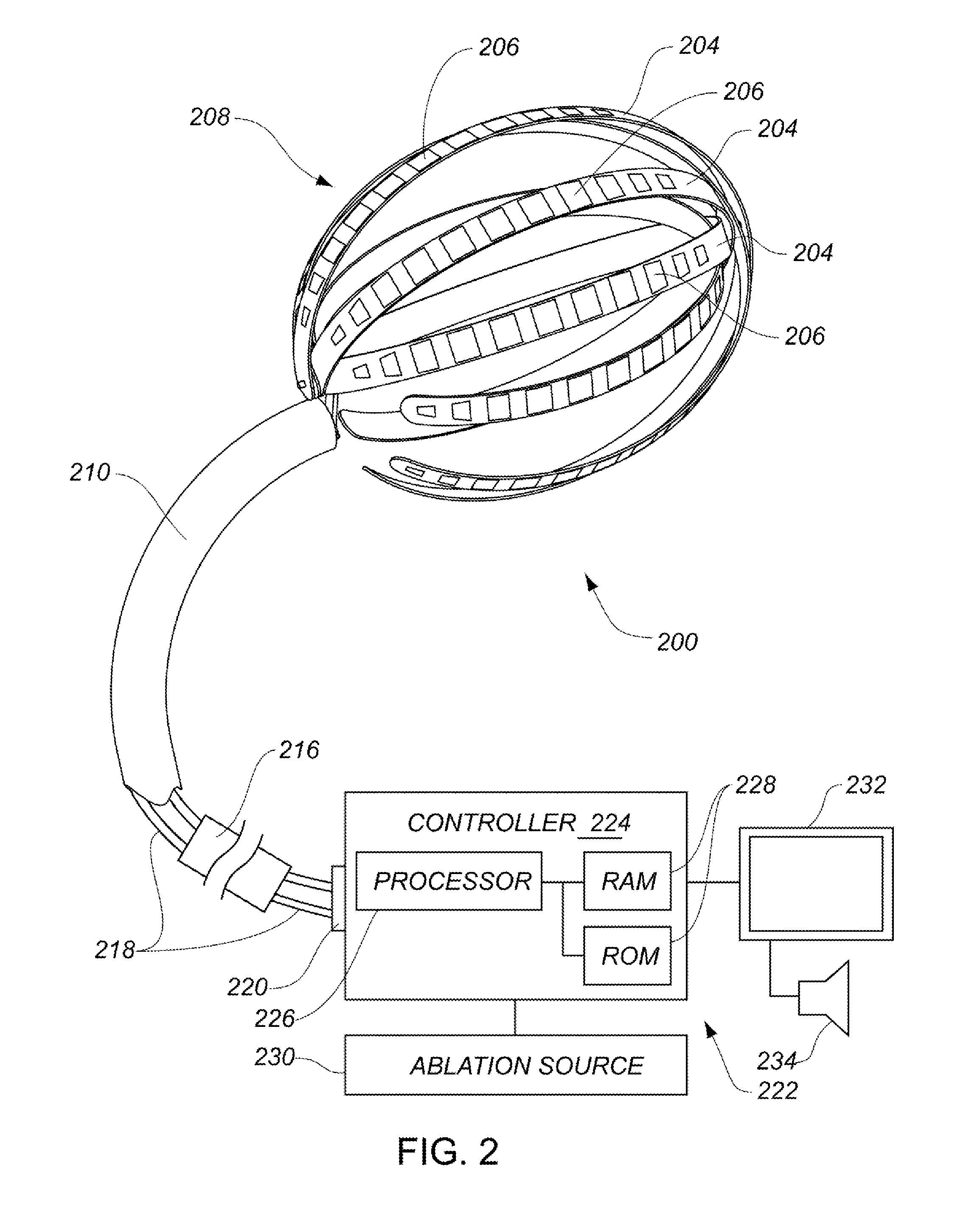
Systems, methods, and devices allow intravascular or percutaneous mapping, orientation or ablation, or combinations thereof in bodily cavities or lumens. A device includes a plurality of elongate members which are moveable between an unexpanded configuration, a bent or coiled stack configuration and an expanded or fanned configuration. The elongate members form a stack arrangement in the unexpanded configuration to fit through a catheter sheath. The elongate members follow respective arcuate or curvilinear paths as advanced from the sheath into the bent or coiled stack configuration, adopting volute, scroll or rho shapes, and may be nested. The elongated members are fanned or radially spaced circumferentially with respect to one another into the expanded or fanned configuration. Transducers carried by elongate members may sense various physiological characteristics of or proximate tissue, for instance temperature, and / or may apply energy to or proximate tissue, for example to perform ablation. The device is retractable.
Owner:KARDIUM
Enhanced medical device for use in bodily cavities, for example an atrium
ActiveUS20130172883A1Enhanced for deployment and positioningImprove ablationElectrocardiographyCatheterProximateTransducer
Systems, methods, and devices allow intravascular or percutaneous mapping, orientation or ablation, or combinations thereof in bodily cavities or lumens. A device includes a plurality of elongate members which are moveable between an unexpanded configuration, a bent or coiled stack configuration and an expanded or fanned configuration. The elongate members form a stack arrangement in the unexpanded configuration to fit through a catheter sheath, The elongate members follow respective arcuate or curvilinear paths as advanced from the sheath into the bent or coiled stack configuration, adopting volute, scroll or rho shapes, and may be nested. The elongated members are fanned or radially spaced circumferentially with respect to one another into the expanded or fanned configuration. Transducers carried by elongate members may sense various physiological characteristics of or proximate tissue, for instance temperature, and / or may apply energy to or proximate tissue, for example to perform ablation. The device is retractable.
Owner:KARDIUM
Enhanced medical device for use in bodily cavities, for example an atrium
ActiveUS20130178851A1Enhanced for deployment and positioningImprove ablationElectrocardiographyCatheterAtrial cavityProximate
Systems, methods, and devices allow intravascular or percutaneous mapping, orientation and / or ablation, in bodily cavities or lumens. A device includes elongate members, moveable between an unexpanded configuration and an expanded or fanned configuration. The elongate members form a stack in the unexpanded configuration to fit through a catheter sheath. The elongate members follow respective arcuate or curvilinear paths as advanced from the sheath into the bent or coiled stack configuration, adopting volute, scroll or rho shapes, and may be nested. The elongated members are fanned or radially spaced circumferentially with respect to one another into the expanded or fanned configuration. Transducer elements carried by elongate members sense various physiological characteristics of or proximate tissue, and / or may apply energy to or proximate tissue. The elongate members are rotatable in groups or as a group in the expanded configuration. The device is retractable.
Owner:KARDIUM
Enhanced medical device for use in bodily cavities, for example an atrium
ActiveUS20130178850A1Enhanced for deployment and positioningImprove ablationElectrocardiographySurgical instrument detailsTransducerMedical device
Systems, methods, and devices allow intravascular or percutaneous mapping, orientation or ablation, or combinations thereof in bodily cavities or lumens. A device includes a plurality of elongate members which are moveable between an unexpanded configuration, a bent or coiled stack configuration and an expanded or fanned configuration. The elongate members form a stack arrangement in the unexpanded configuration to fit through a catheter sheath, The elongate members follow respective arcuate or curvilinear paths as advanced from the sheath into the bent or coiled stack configuration, adopting volute, scroll or rho shapes, and may be nested. The elongated members are fanned or radially spaced circumferentially with respect to one another into the expanded or fanned configuration. Transducers carried by elongate members may sense various physiological characteristics of or proximate tissue, for instance temperature, and / or may apply energy to or proximate tissue, for example to perform ablation. The device is retractable.
Owner:KARDIUM
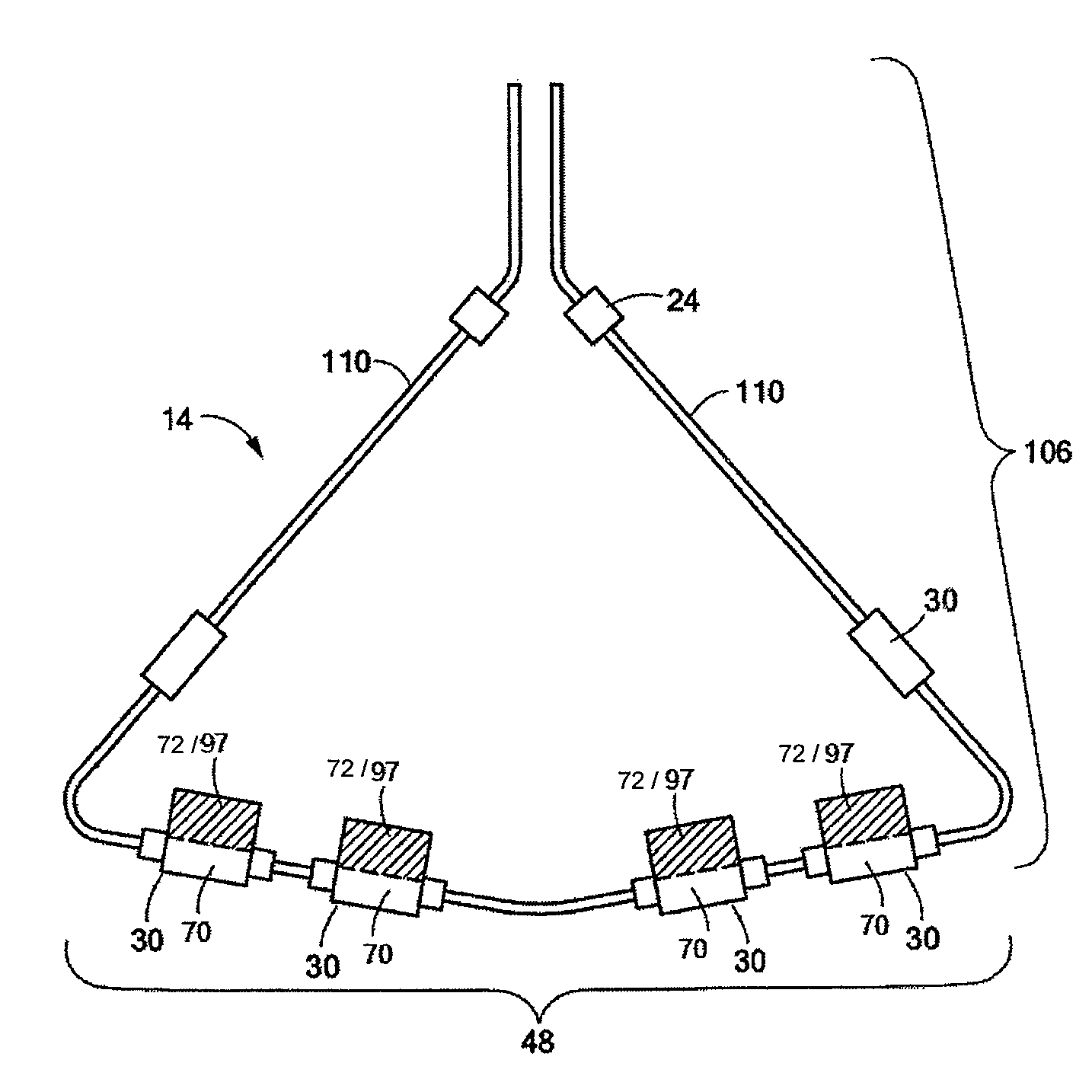
Intra-cardiac mapping and ablating
ActiveUS20130066220A1Improve abilitiesImprove ablation positioningCatheterSurgical instrument detailsBlood flowTransducer
Systems, methods, and devices allow percutaneous mapping, orientation and / or ablation in bodily cavities or lumens. Such may include a structure that is percutaneously positionable in a cavity, such as an intra-cardiac cavity of a heart. Transducers carried by the structure are responsive to blood flow. For example, the transducers may sense temperature, temperature being related to convective cooling caused by blood flow. A controller discerns positional information or location, based on signals from the transducers. For example, blood flow may be greater and / or faster proximate a port in cardiac tissue than proximate tissue spaced from the port. Position information may allow precise ablation of selected tissue, for example tissue surround a port in the intra-cardiac cavity.
Owner:KARDIUM
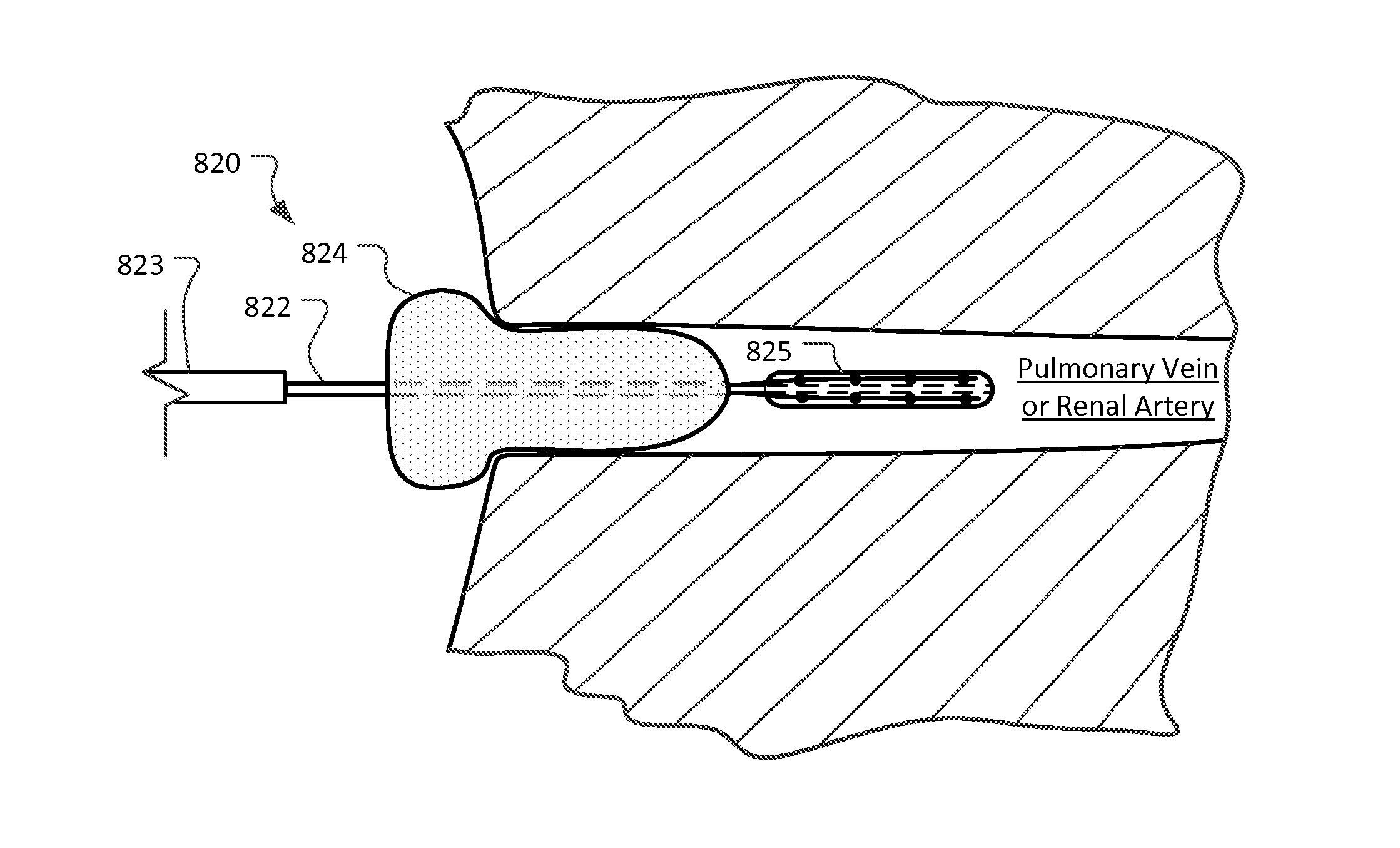
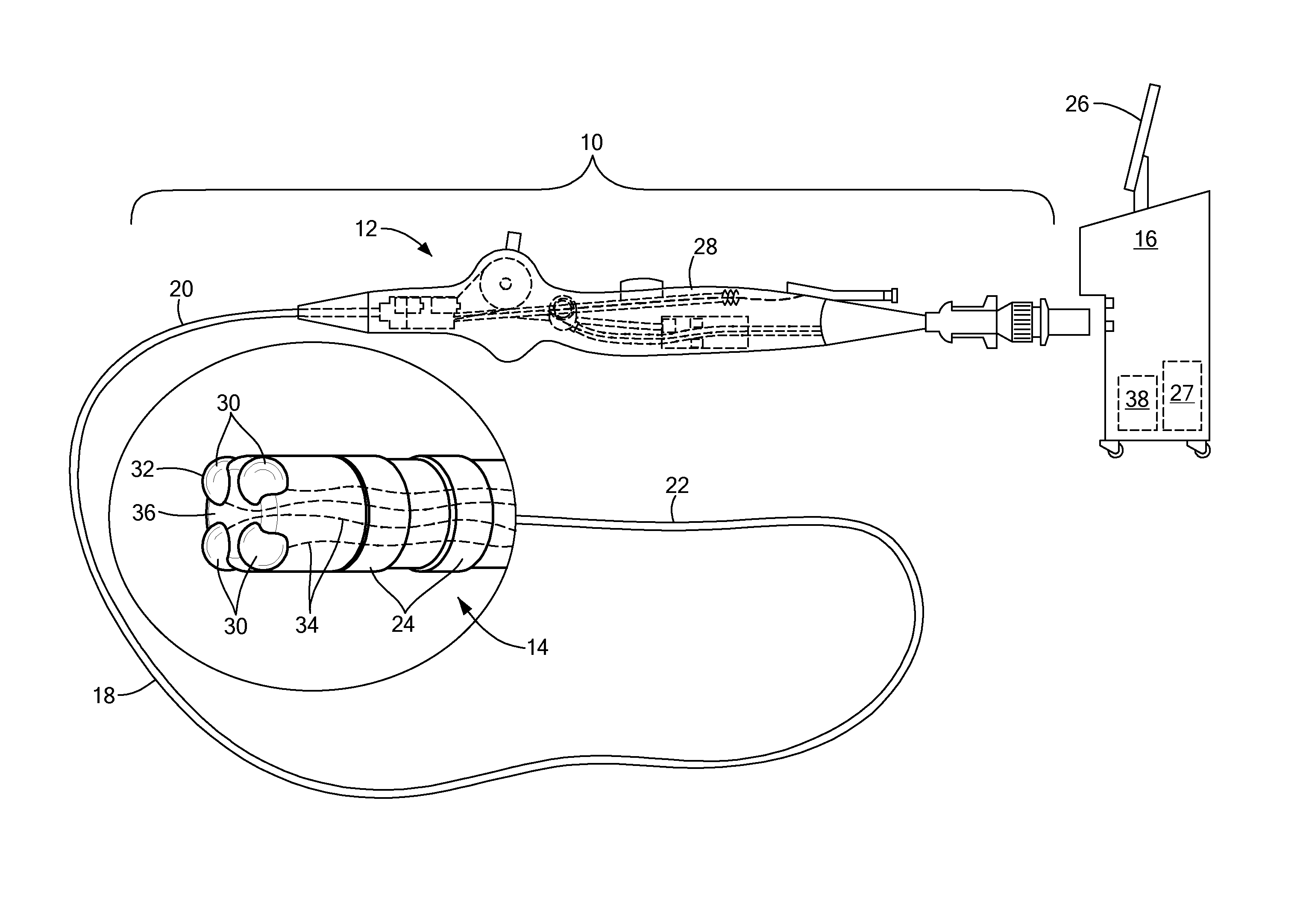
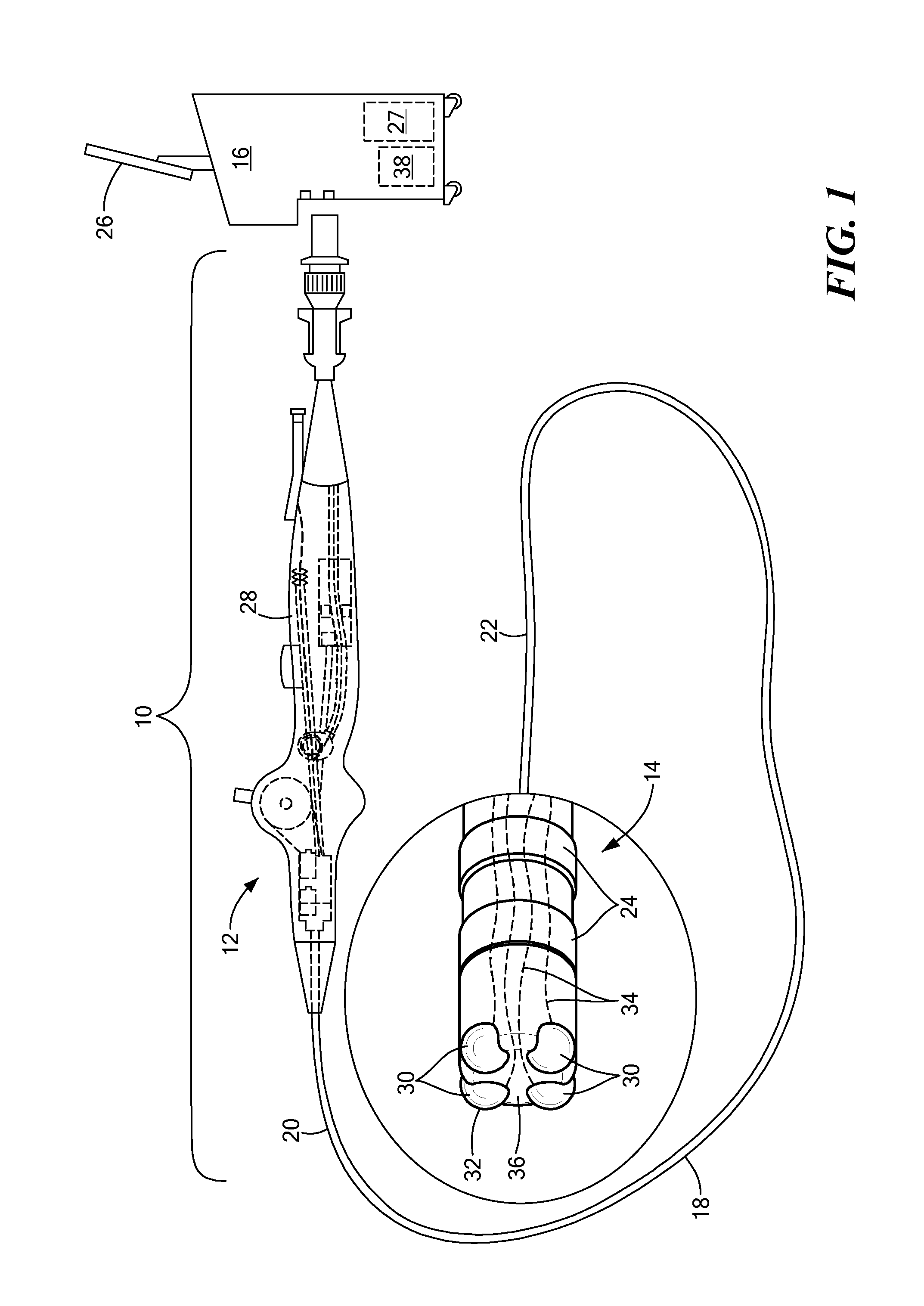
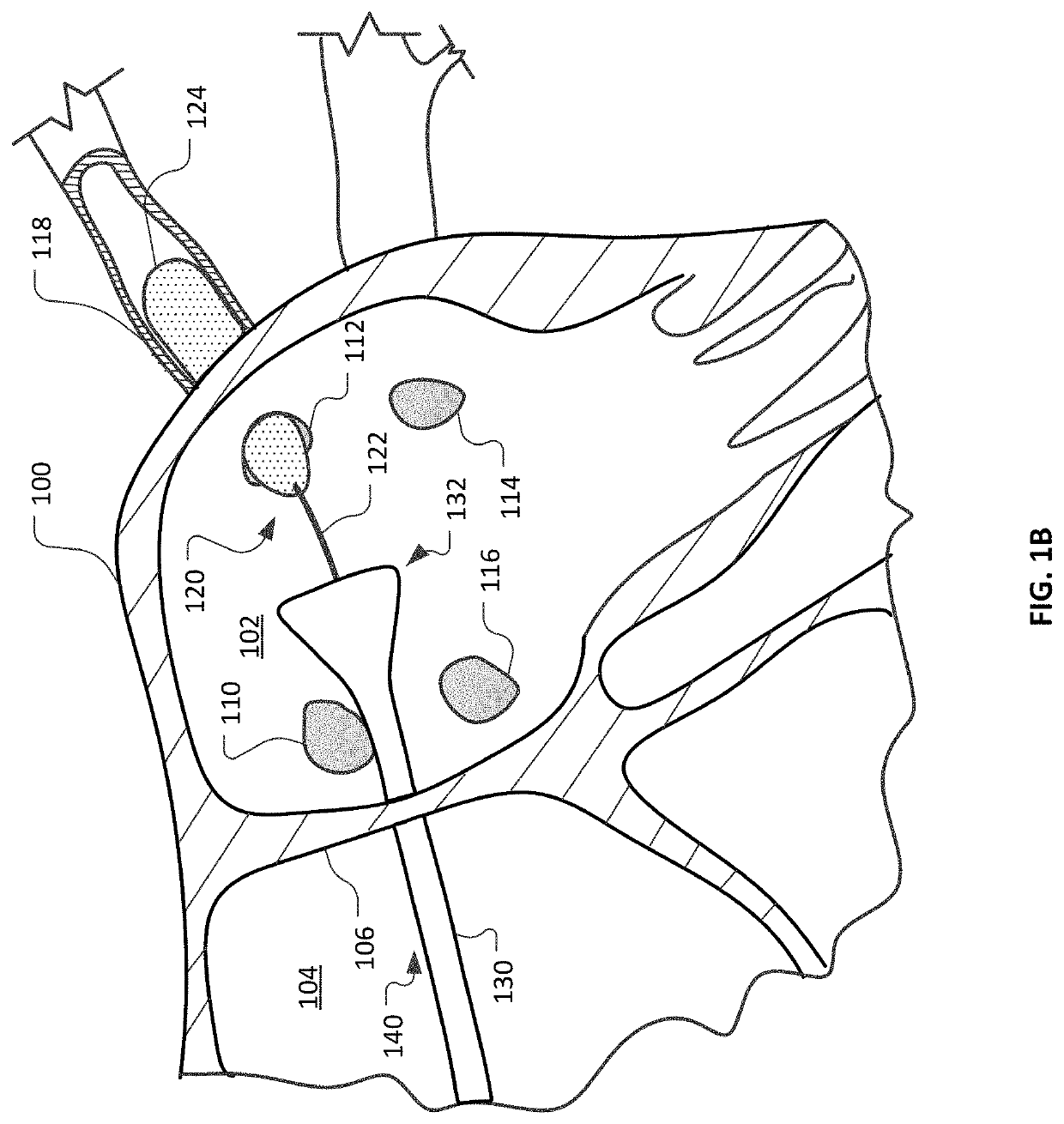
Devices and methods for ablation of tissue
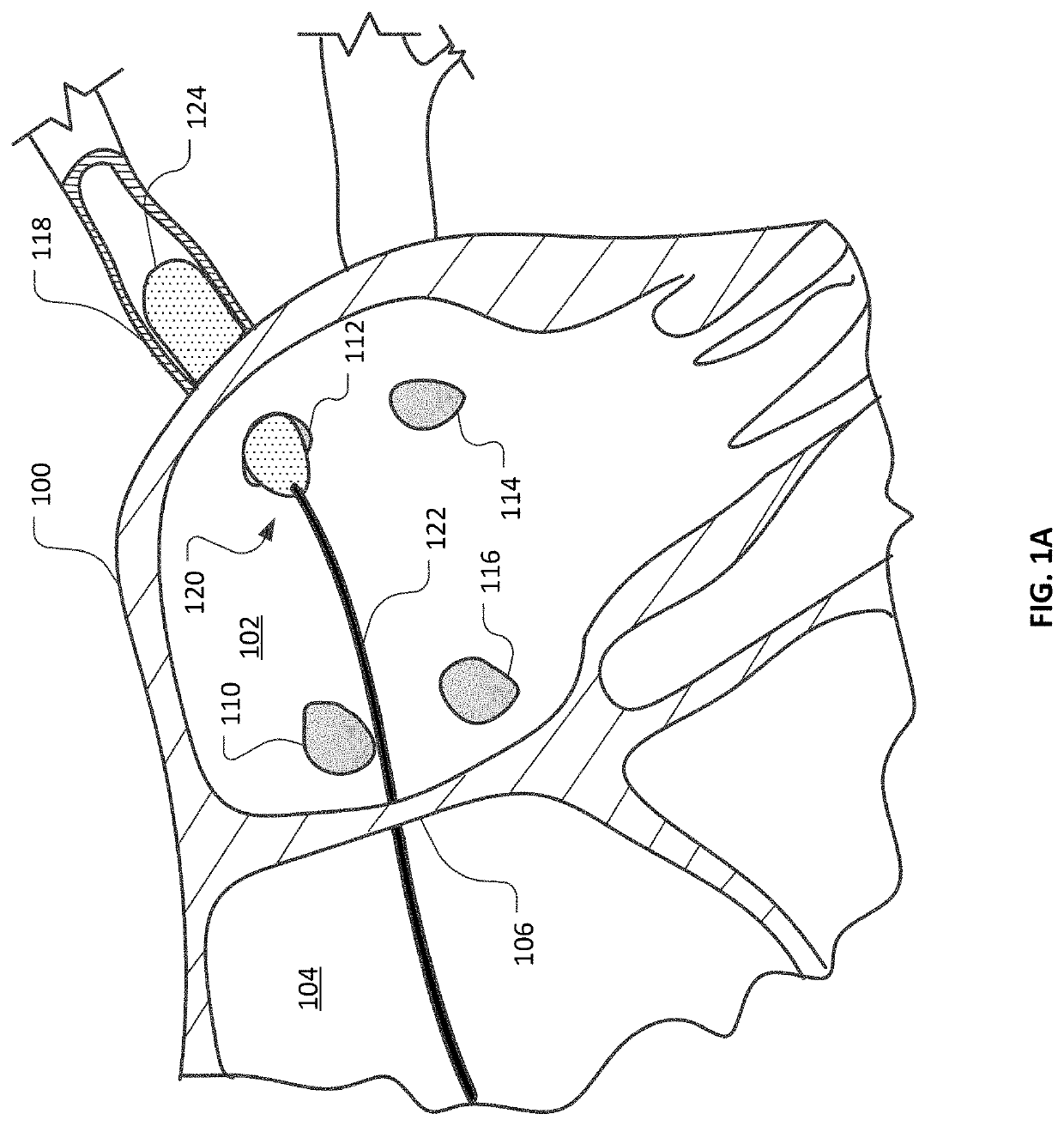
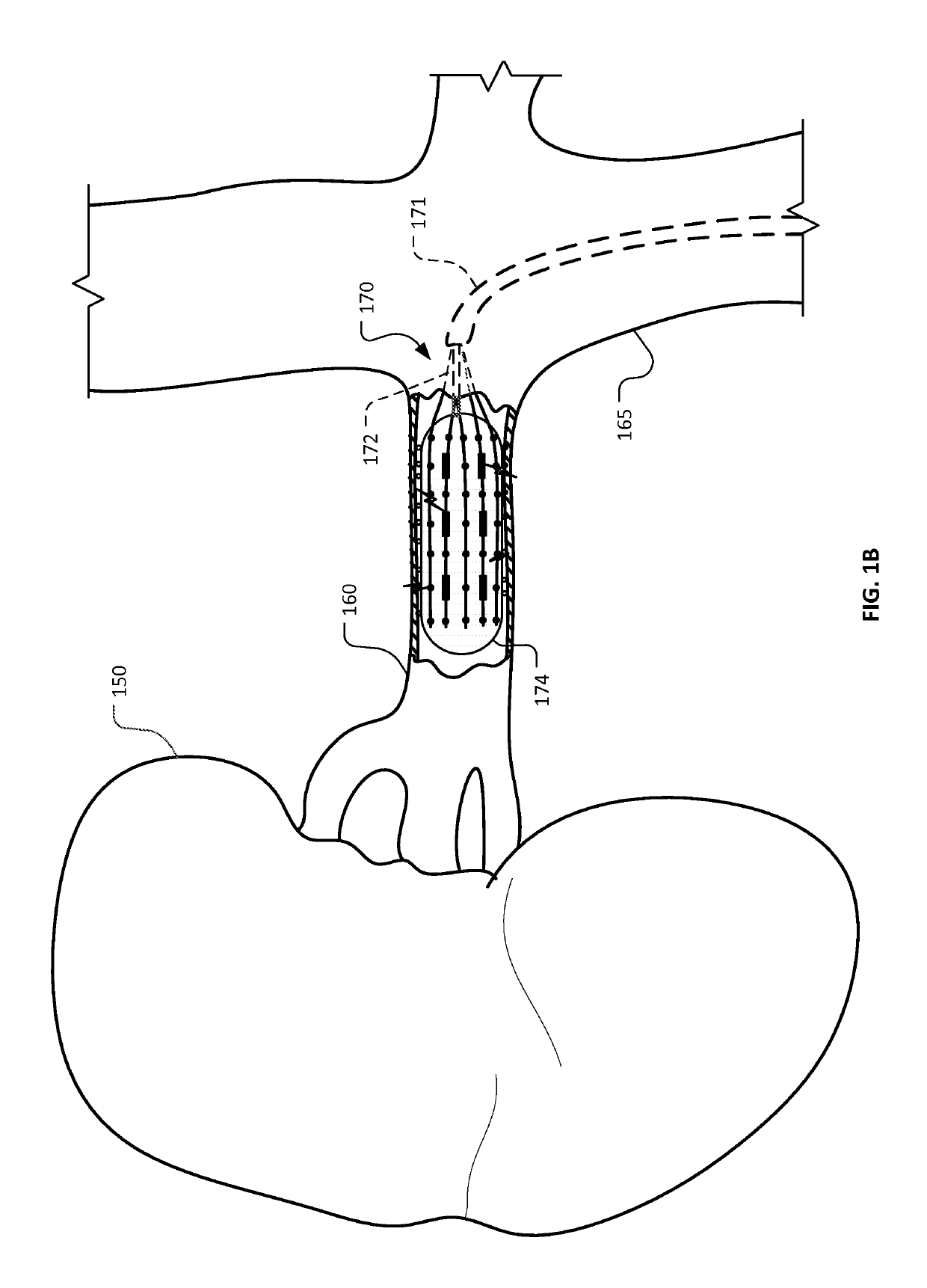
ActiveUS20160374754A1Avoid flowFacilitate ablationElectrotherapyBalloon catheterDiseaseMedical disorder
This document provides devices and methods for the treatment of heart conditions, hypertension, and other medical disorders. For example, this document provides devices and methods for treating atrial fibrillation by performing thoracic vein ablation procedures, including pulmonary vein myocardium ablation. In some embodiments, the ablation is performed in coordination with the delivery a pharmacological agent that can abate the formation of tissue stenosis or neointimal hyperplasia caused by the ablation.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Intra-cardiac mapping and ablating
ActiveUS9277960B2Improve abilitiesConvenient location informationCatheterSurgical instruments for heatingBlood flowTransducer
Owner:KARDIUM
Electrophysiology catheter design
ActiveUS9370311B2Improved mappingImprove ablationElectrocardiographyContact member assembly/disassemblyElectrophysiologyBiomedical engineering
Owner:MEDTRONIC ABLATION FRONTIERS
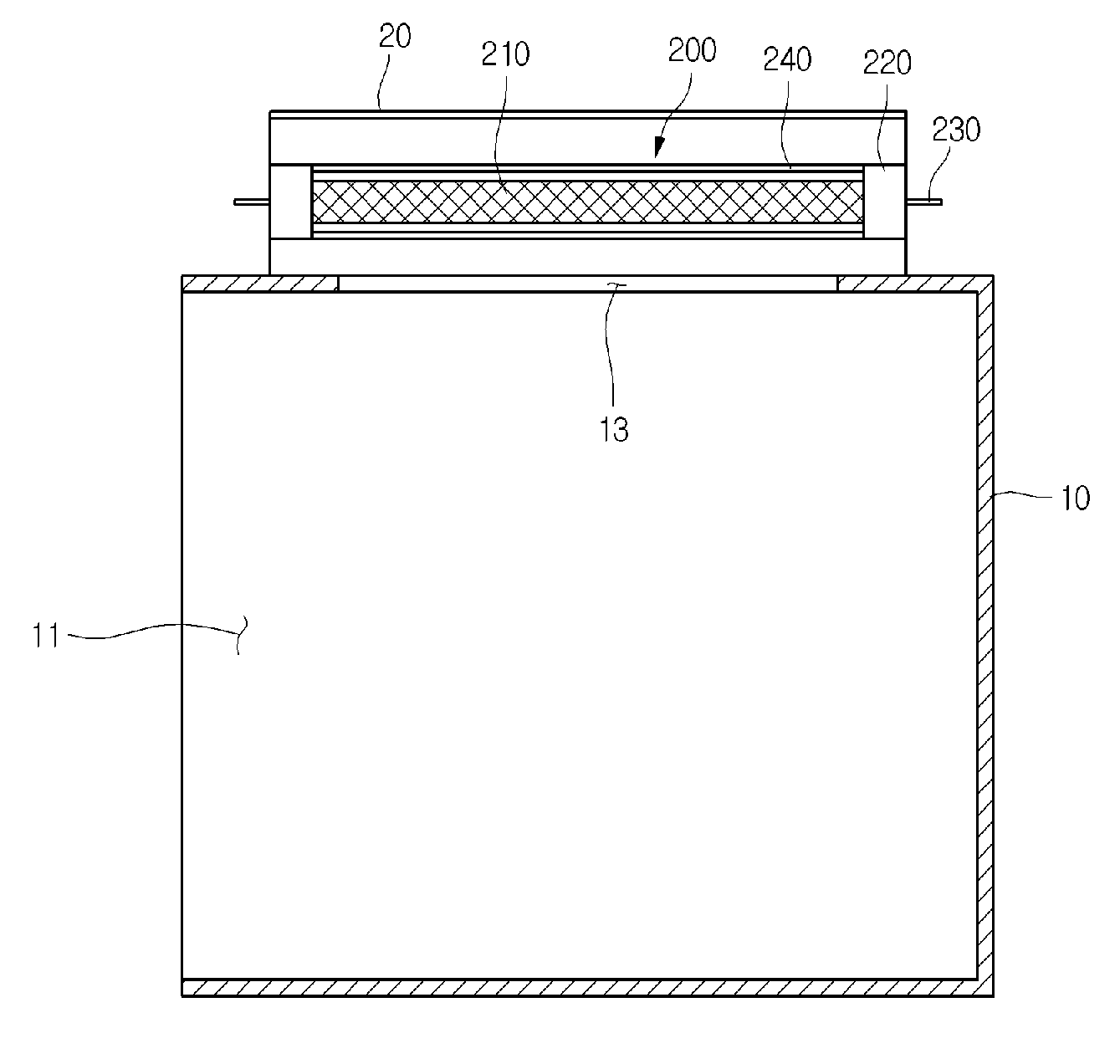
Interstitial ultrasonic disposable applicator and method for tissue thermal conformal volume ablation and monitoring the same
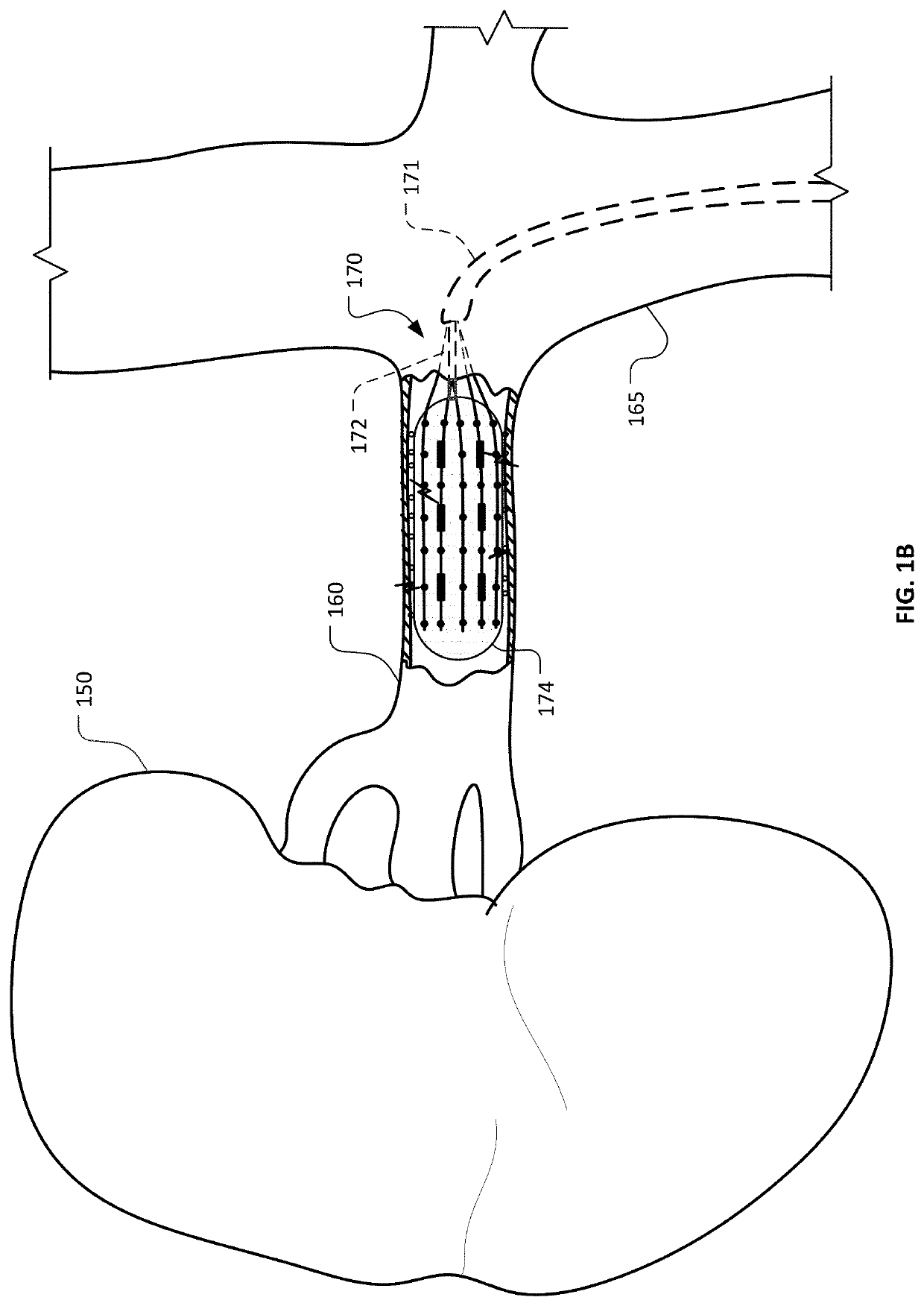
InactiveUS20140257262A1Improve ablationSolve the uneven volumeUltrasound therapySurgical instruments for heatingSonificationMedicine
An interstitial ultrasound thermal ablation applicator for conformal treatment of inhomogeneous tumor lesions includes: a body having a longitudinal axis, the body defining a hollow central channel along the longitudinal axis; and a plurality of CMUT array transducers mounted on the body, arranged side by side to form a cylindrical shape, having azimuth plans parallel to a longitudinal axis of the body, each of the plurality of CMUT array transducers having elevation dimensions predetermined to steer emitted ultrasonic waves to obtain a conformal volume treatment of the tumor lesions. An electronic driving method for driving an applicator having multiple independent transducer elements arranged in rows and columns includes: controlling focal parameters of each row and column of transducer elements; and controlling a contribution of each row and column of transducer elements in a manner to provide a conformal ablated volume.
Owner:VERMON +3
Electrophysiology catheter design
ActiveUS20140052119A1Improved mappingImprove ablationContact member assembly/disassemblyMagnetic/electric field screeningPower flowElectrophysiology
The present invention relates to a method, device, and system for improved mapping and / or ablation of a tissue. The device may generally include an elongate body and a distal assembly affixed to the elongate body that includes a treatment electrode having a conductive mapping region and a selectively conductive ablation region that is conductive of high-frequency current and substantially non-conductive of low-frequency current. Alternatively, the device may generally include a treatment electrode having a conductive mapping or ablation region and a region that is coated with an electrically insulated but thermally conductive layer.
Owner:MEDTRONIC ABLATION FRONTIERS
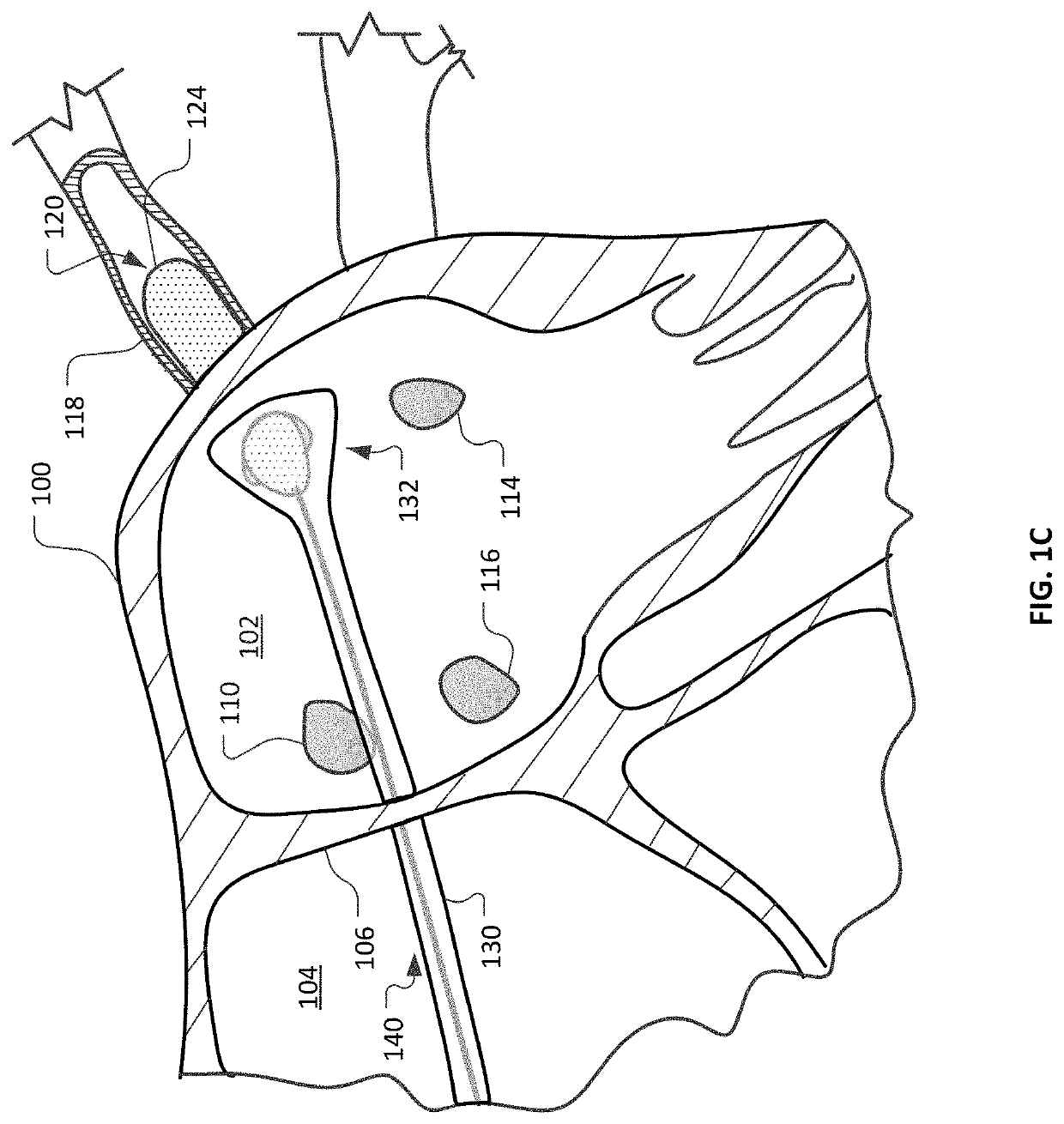
Devices and methods for ablation of tissue
InactiveUS20180360531A1Facilitate ablationPrevent blood flowBalloon catheterMedical devicesMedical disorderVein
Devices and methods for the treatment of heart conditions, hypertension, and other medical disorders are described. For example, this document describes devices and methods for treating atrial fibrillation by performing thoracic vein ablation procedures, including pulmonary vein myocardium ablation. In some embodiments, the ablation is performed in coordination with the delivery a pharmacological agent that can abate the formation of tissue stenosis or neointimal hyperplasia caused by the ablation. Additionally, in some embodiments, particulate matter, such as thrombus or crystalline drug compounds, created during the ablation is captured and removed from the patient using devices and methods provided herein. Further, devices and methods for non-thermal methods of causing cell death, such as tissue suction and tissue stretching, are also described.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Adjustable length and/or exposure electrodes
An ablation system is provided. The ablation system includes an ablation device having a handle assembly and an electrode assembly. The handle assembly includes a housing configured for operable engagement by a user. The electrode assembly includes a tubular electrode having an open proximal end and a closed distal end. The tubular electrode is movable relative to the housing to adjust an insertable length of the electrode assembly.
Owner:TYCO HEALTHCARE GRP LP
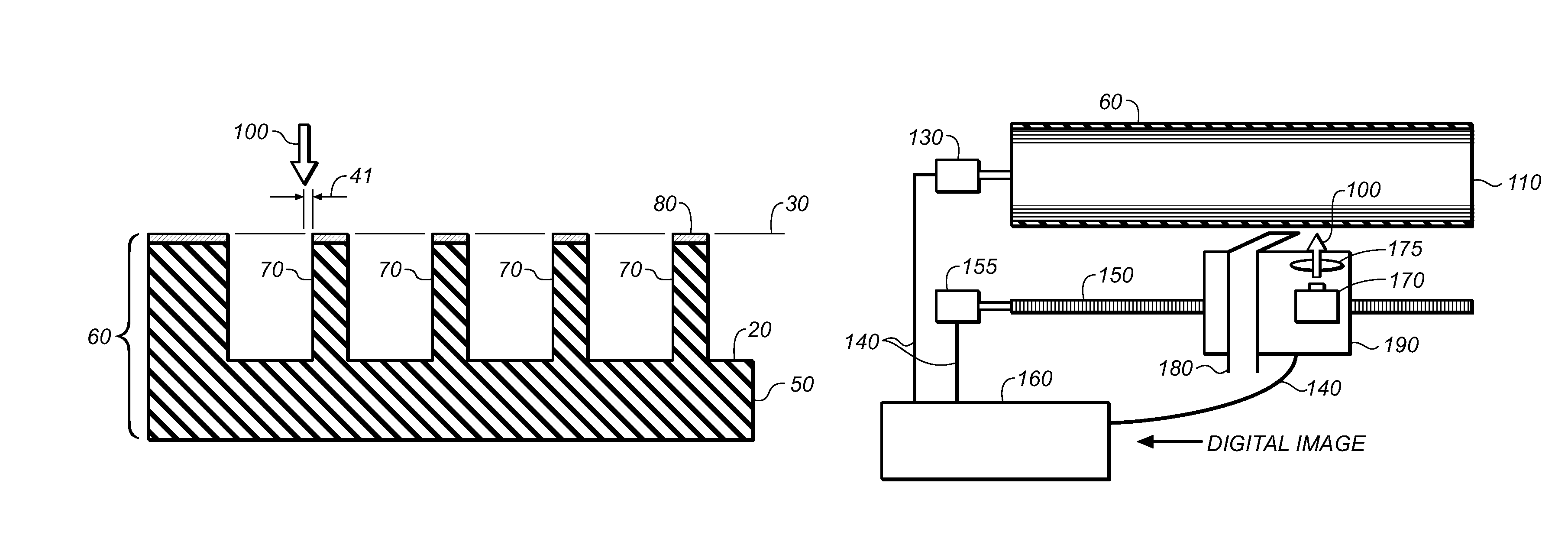
Method for direct engraving of flexographic printing members
InactiveUS20130269557A1Maximize efficiencyImprove heat uniformityPlate printingFoil printingEngravingControl layer
A method for engraving a flexographic relief member includes providing a laser engraveable flexographic member; providing a thin engraveable control layer on top of the laser and engraveable flexographic member; the flexographic relief member comprises the laser engraveable flexographic member and the thin engraveable control layer; the engraveable control layer has an engraving sensitivity lower than the flexographic member; and scanning a radiation beam on the flexographic relief member to engrave the flexographic relief member.
Owner:EASTMAN KODAK CO
Medical device for use in bodily lumens, for example an atrium
ActiveUS20140214028A1Increase capacityConvenient location informationDiagnostic recording/measuringSensorsProximateFluid tissues
A device positionable in a cavity of a bodily organ (e.g., a heart) may discriminate between fluid (e.g., blood) and non-fluid tissue (e.g., wall of heart) to provide information or a mapping indicative of a position and / or orientation of the device in the cavity. Discrimination may be based on flow, or some other characteristic, for example electrical permittivity or force. The device may selectively ablate portions of the non-fluid tissue based on the information or mapping. The device may detect characteristics (e.g., electrical potentials) indicative of whether ablation was successful. The device may include a plurality of transducers, intravascularly guided in an unexpanded configuration and positioned proximate the non-fluid tissue in an expanded configuration. Expansion mechanism may include helical member(s) or inflatable member(s).
Owner:KARDIUM
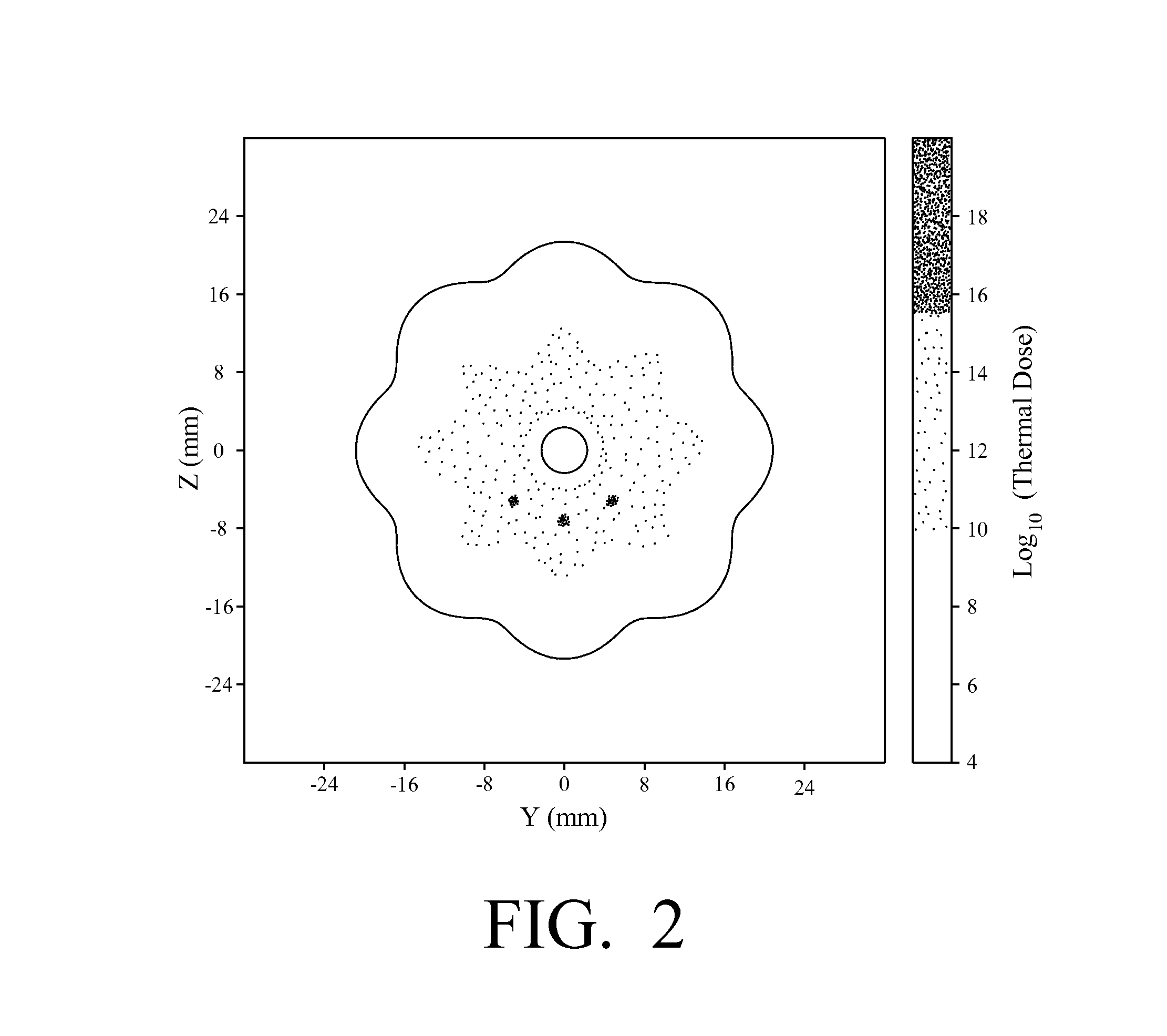
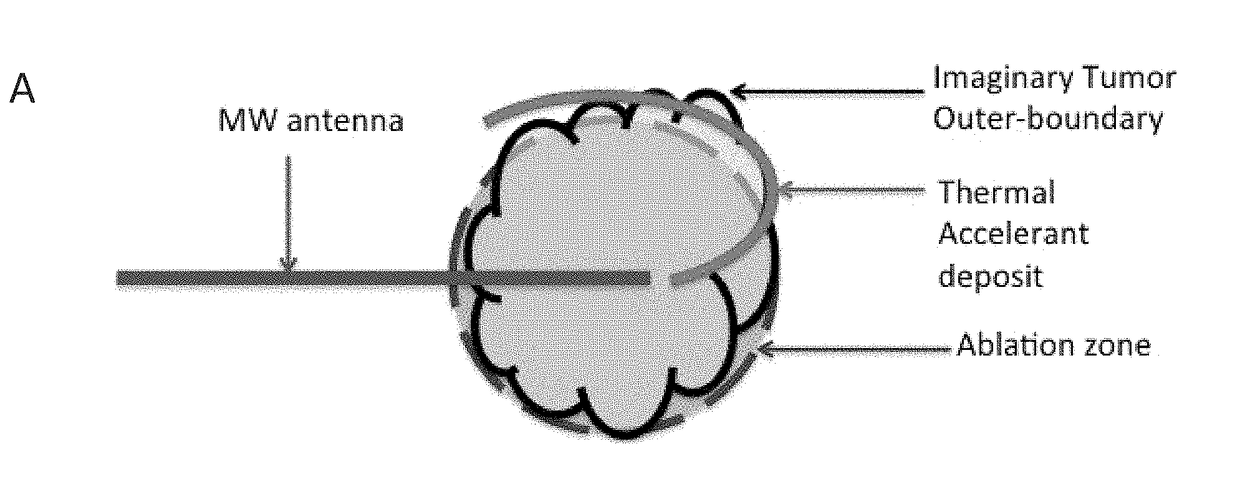
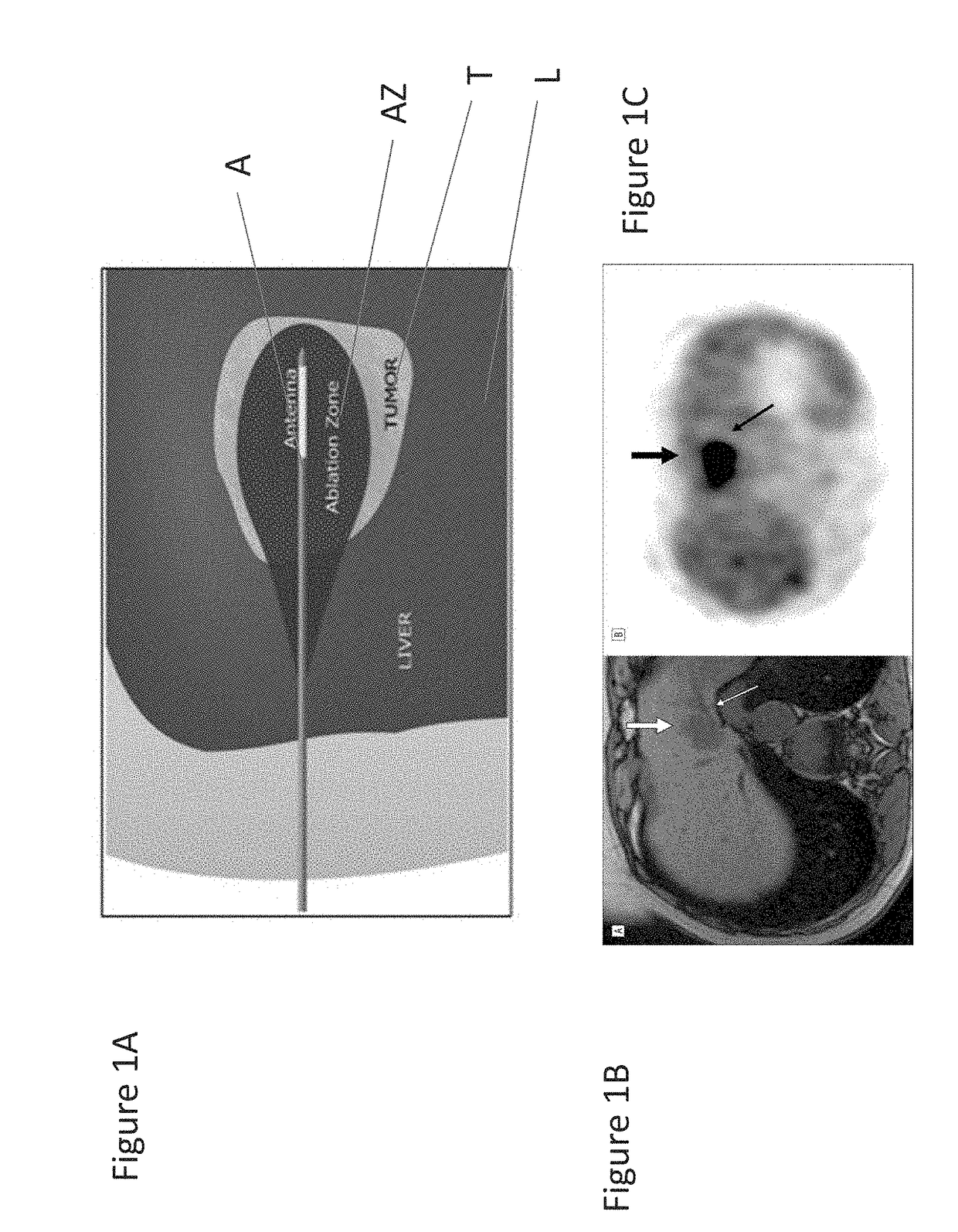
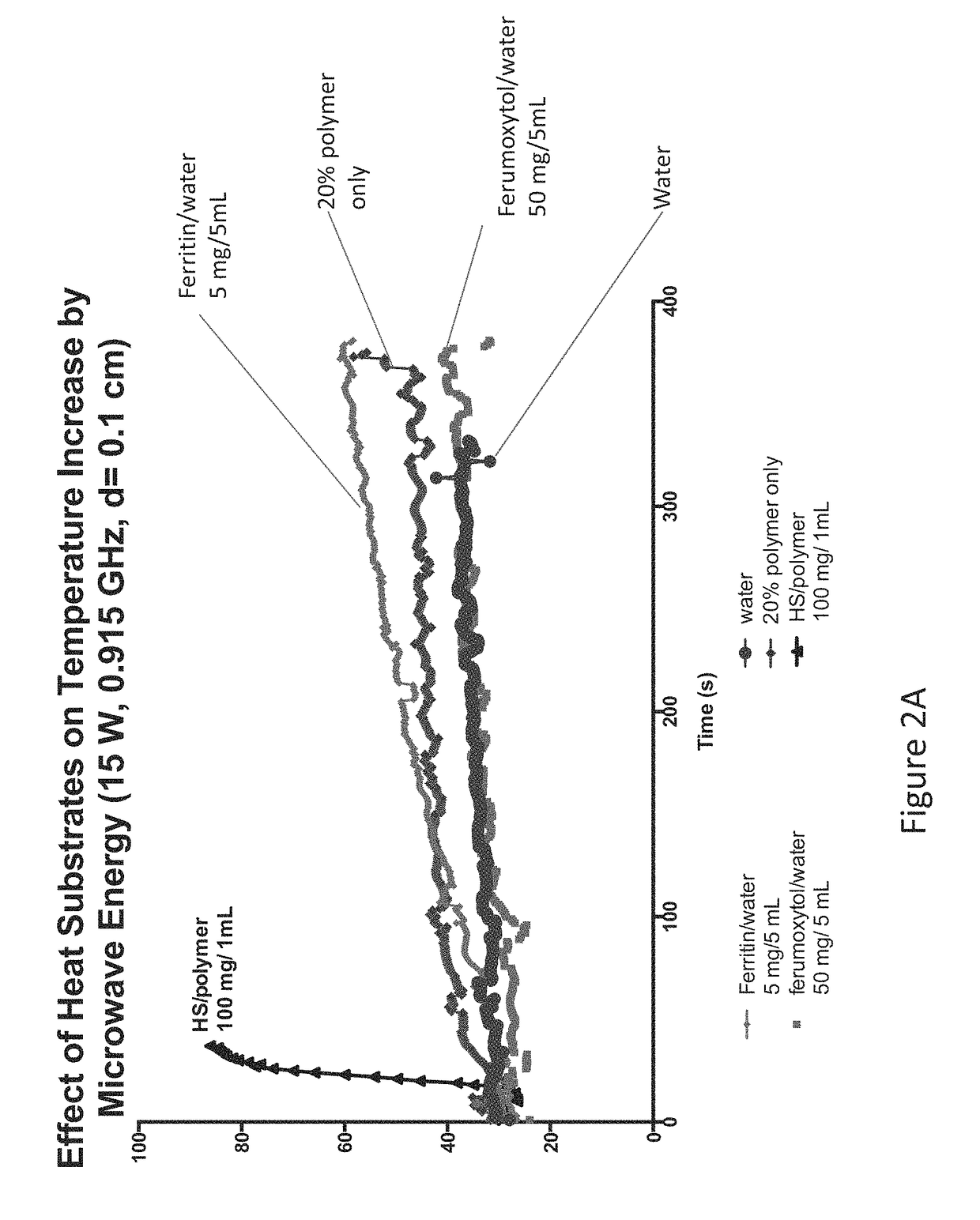
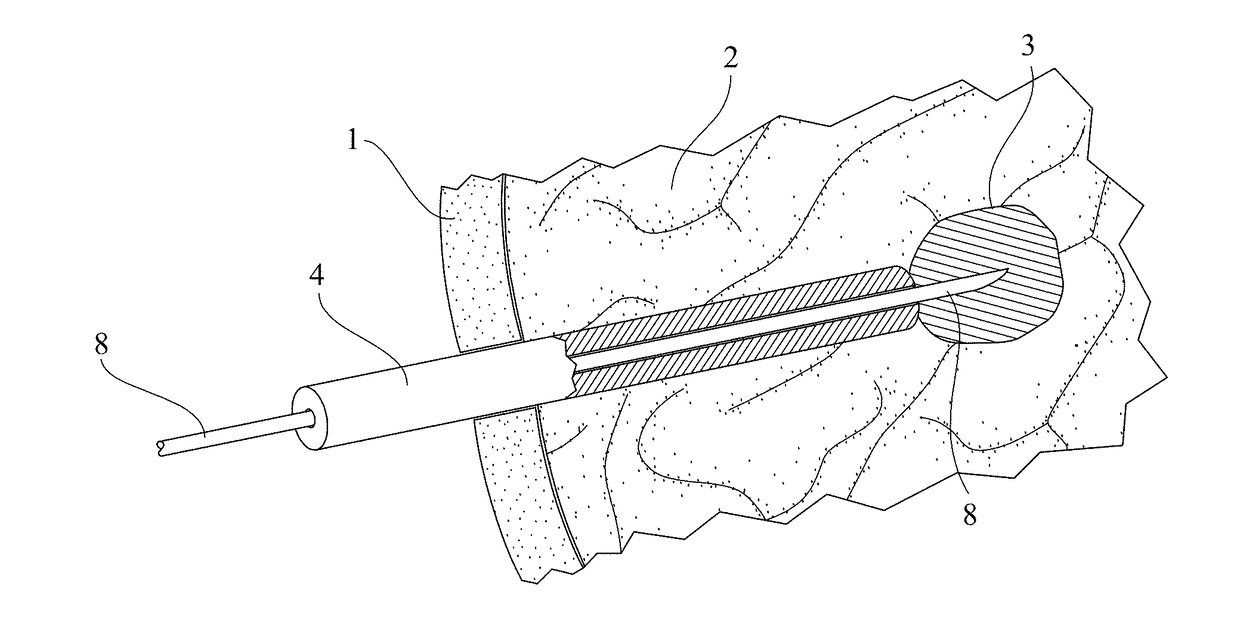
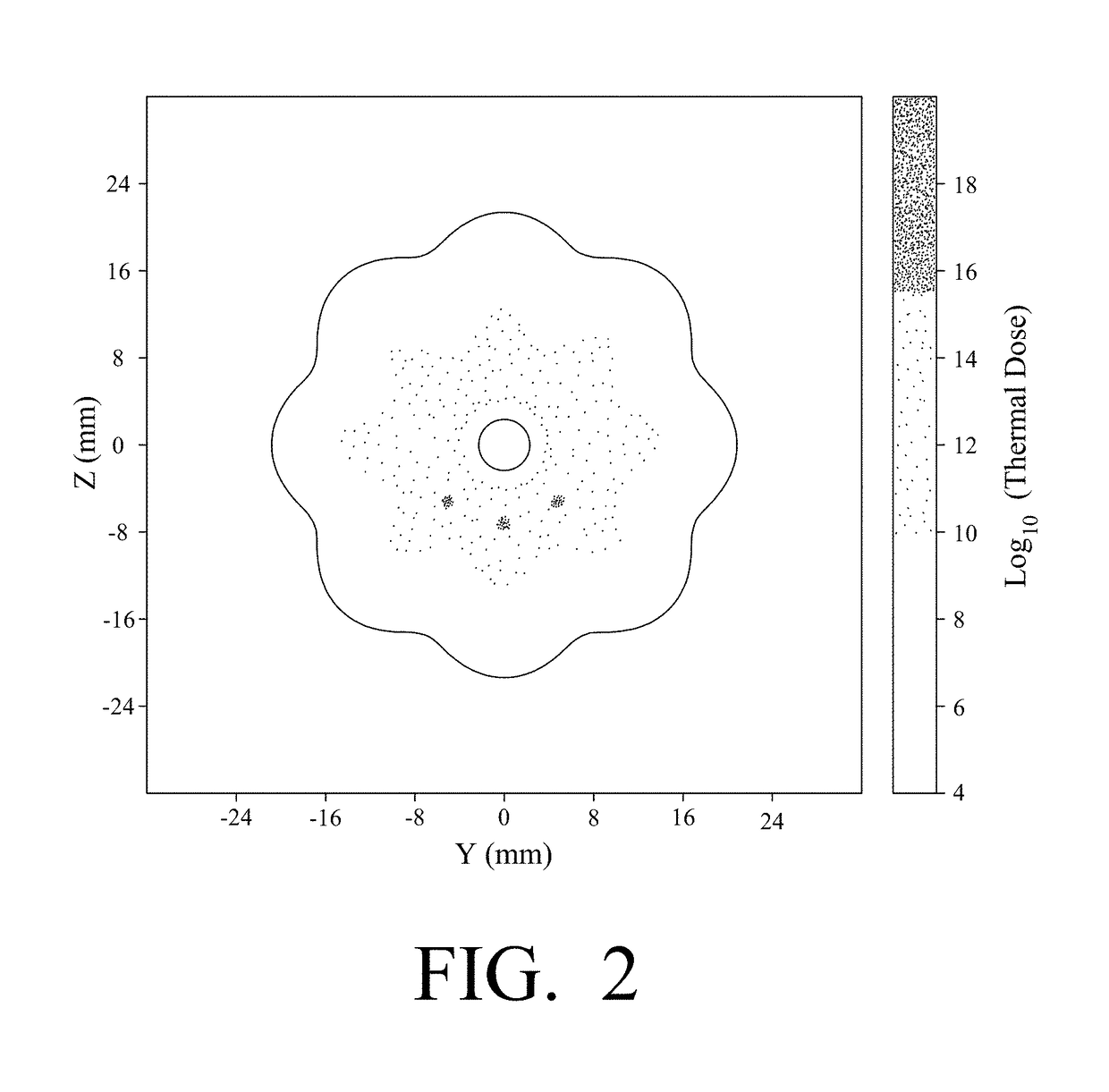
Thermal accelerant compositions and methods of use
ActiveUS20170182165A1Efficient heatingImprove completenessElectrotherapyEnergy modified materialsMicrowaveHeat sensitive
A thermal accelerant is delivered to a tissue site and localized to modulate the shape, extent or other characteristic of RF or microwave-induced hyperthermic tissue ablation. The accelerant may be provided via an image-guided hand piece or via a lumen added to a microwave antenna, and promotes faster heating, more complete ablation and / or a more extensive treatment region to reduce recurrence of treated cancers, overcoming natural limitations, variations in tissue response and drop-off or thermal loss away from the antenna. The accelerant is delivered as a low-viscosity but heat sensitive fluid, and is fixed in place to provide regions of preferential absorption or heating. Shorter exposure times to heat the far field may allow survival of vulnerable tissue such as vessels, and multiple antennae may be used for effective treatment of irregular or large tumors.
Owner:RHODE ISLAND HOSPITAL +1
Interstitial ultrasonic disposable applicator and method for tissue thermal conformal volume ablation and monitoring the same
An interstitial ultrasound thermal ablation applicator for conformal treatment of an inhomogeneous tumor lesion includes: a body having a longitudinal axis; and a plurality of array transducers mounted on the body, arranged side by side and having azimuth directions parallel to the longitudinal axis of the body, and having outer faces disposed in a polygonal arrangement; the plurality of array transducers having predetermined elevation dimensions defined for directing emitted ultrasonic energy to obtain a conformal volume treatment of the tumor lesions. An electronic driving method for driving an applicator having multiple independent transducer elements arranged in rows and columns includes: controlling focal parameters of each row and column of transducer elements; and controlling a contribution of each row and column of transducer elements in a manner to provide a conformal ablated volume.
Owner:INST NAT DE LA SANTE & DE LA RECH MEDICALE INSERM U1032 +2
Method for coating oxidation protective layer for carbon/carbon composite, carbon heater, and cooker
ActiveUS20130075387A1OxidationHigh thermal stability resistanceOhmic-resistance electrodesPretreated surfacesFiberCarbon composites
The present invention provides an oven range. In the present invention, a method for coating an oxidation protective layer for a carbon / carbon composite, comprising: dissolving a polymer resin in a solvent to form a solution of polymer resin; dispersing ceramic powders in the solution of polymer resin to form a mixed solution; coating the mixed solution on a carbon fiber; performing a first heat-treatment to treat the carbon fiber by heat in air; and performing a second heat-treatment to treat the carbon fiber by heat under an inert gas.
Owner:LG ELECTRONICS INC
System for direct engraving of flexographic printing members
InactiveUS20130270236A1Maximize efficiencyImprove heat uniformityAuxillary shaping apparatusPlate printingControl layerLaser scanning
A system for engraving a flexographic relief member includes a laser scanning apparatus providing a focused radiation beam. The flexographic relief member includes a laser engravable flexographic member; a thing engravable control layer on top of the flexographic member; and wherein the engravable control layer has an engraving sensitivity lower than the flexographic member.
Owner:MIRACLON CORP
Devices and methods for ablation of tissue
ActiveUS20190336208A1Avoid flowFacilitate ablationElectrotherapyBalloon catheterMedical disorderDisease
This document provides devices and methods for the treatment of heart conditions, hypertension, and other medical disorders. For example, this document provides devices and methods for treating atrial fibrillation by performing thoracic vein ablation procedures, including pulmonary vein myocardium ablation. In some embodiments, the ablation is performed in coordination with the delivery a pharmacological agent that can abate the formation of tissue stenosis or neointimal hyperplasia caused by the ablation.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
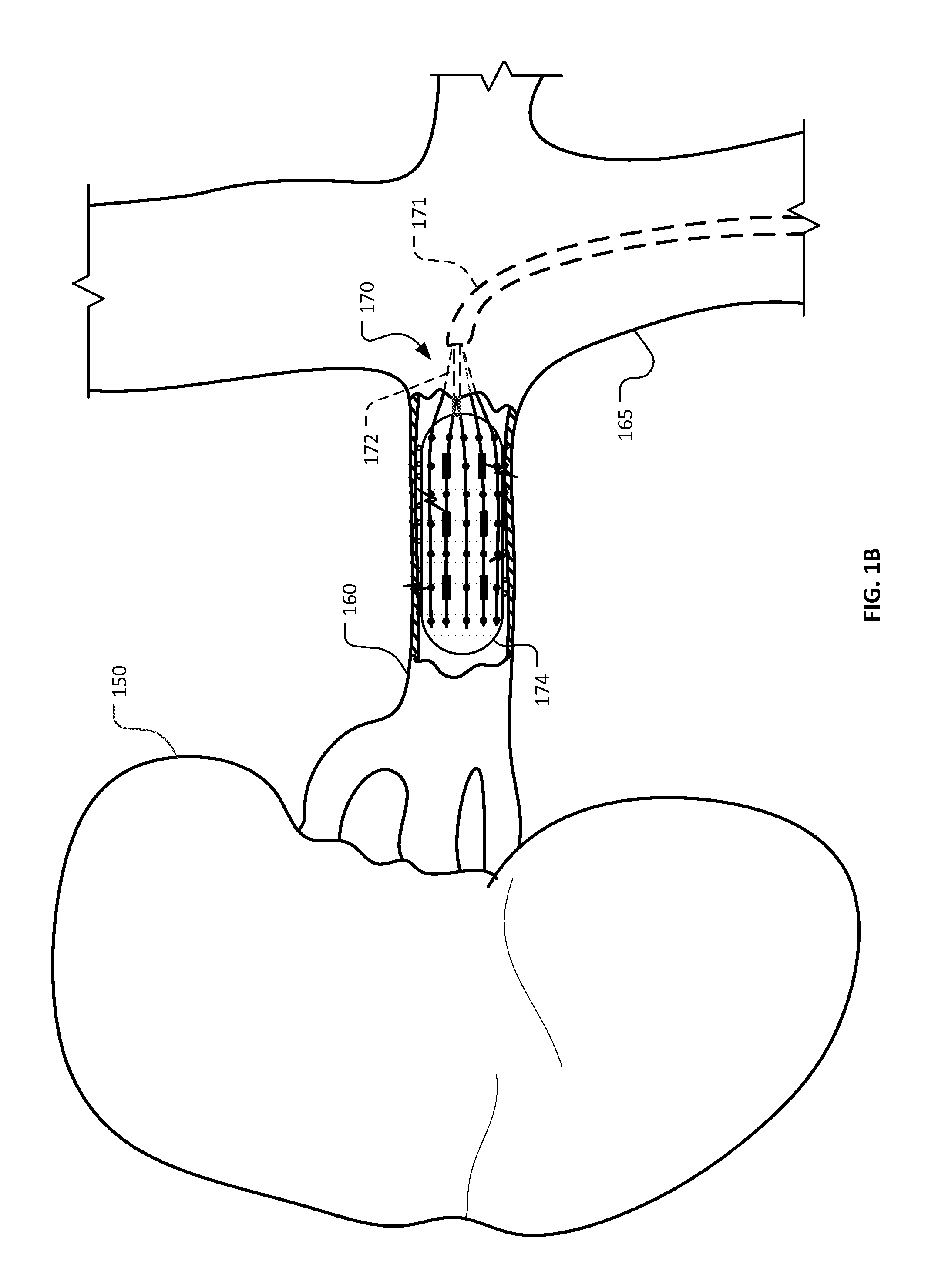
Mapping ablation catheter
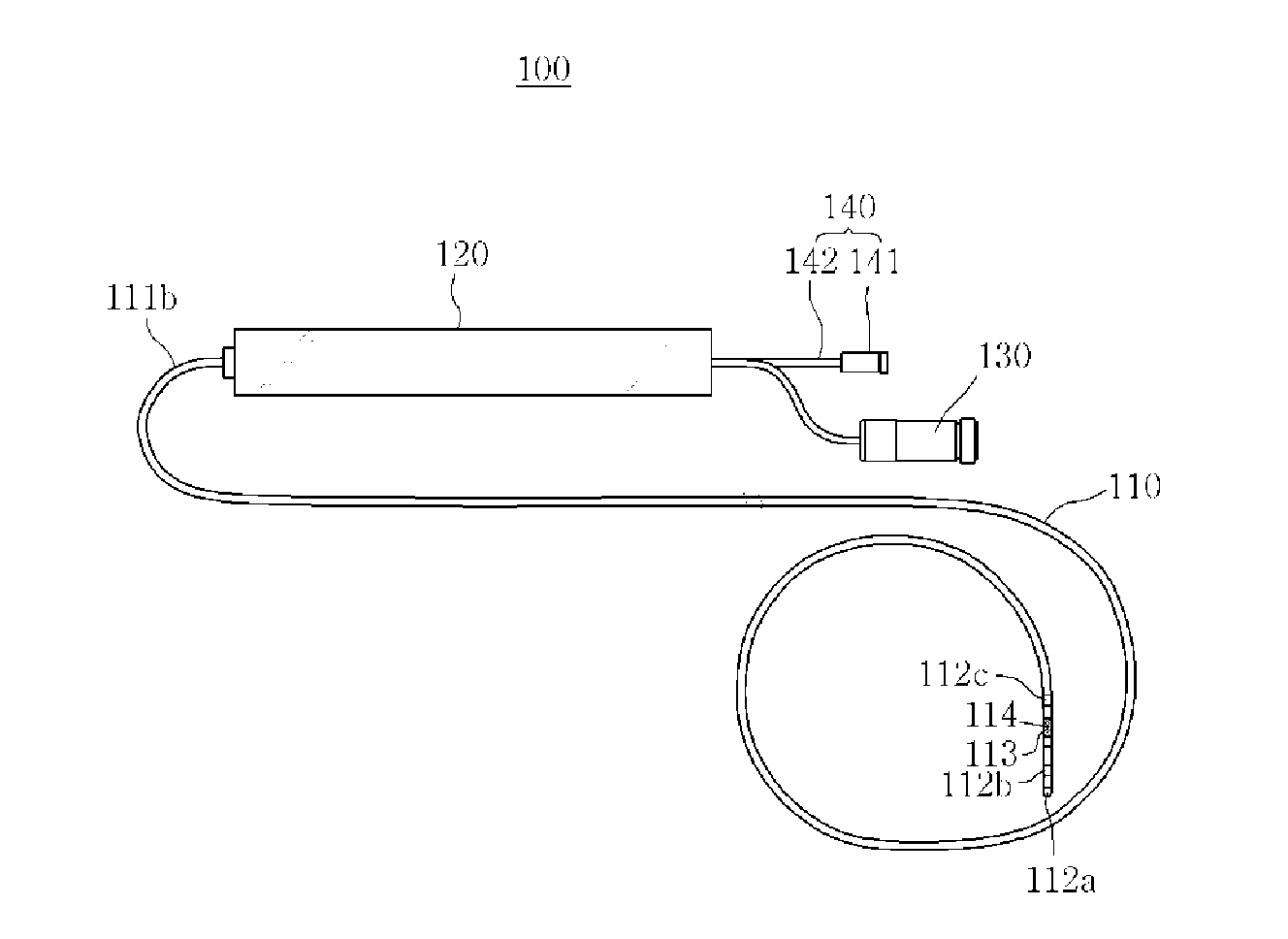
ActiveUS20160008060A1Increase contact areaCurrent providedElectrocardiographyCatheterPower flowMedicine
A mapping ablation catheter is provided comprising: a catheter conduit having a hollow inner space in which a guide member is disposed; a monitoring electrode unit arranged to surround the outer circumferential surface of the distal portion of the catheter conduit and perform a mapping on the lesion region by being in side-contact with a cardiac organ; an ablation electrode unit arranged to be spaced apart from the monitoring electrode unit and surround the outer circumferential surface of the distal portion of the catheter conduit and which removes the lesion region; a plurality of liquid discharge holes arranged on the outer circumferential surface of the ablation electrode; a current application unit which applies current to the monitoring electrode unit and the ablation electrode unit; and a liquid supply unit having a liquid supply tube, one end of the liquid-supply tube connected to the liquid discharge holes in the catheter conduit.
Owner:KOREA UNIV RES & BUSINESS FOUND
Laser-engraveable compositions and flexographic printing precursors
ActiveUS8603725B2Achieve recyclabilityImprove the immunityPhotosensitive materialsLayered productsPorous particleOrganic chemistry
Owner:EASTMAN KODAK CO
Electrophysiology catheter design
ActiveUS20160262831A1Improved mappingImprove ablationContact member assembly/disassemblyMagnetic/electric field screeningPower flowElectrophysiology
The present invention relates to a method, device, and system for improved mapping and / or ablation of a tissue. The device may generally include an elongate body and a distal assembly affixed to the elongate body that includes a treatment electrode having a conductive mapping region and a selectively conductive ablation region that is conductive of high-frequency current and substantially non-conductive of low-frequency current. Alternatively, the device may generally include a treatment electrode having a conductive mapping or ablation region and a region that is coated with an electrically insulated but thermally conductive layer.
Owner:MEDTRONIC ABLATION FRONTIERS
Devices and methods for ablation of tissue
PendingUS20220346870A1Facilitate ablationImprove ablationBalloon catheterMedical devicesDiseaseNeointima
Devices and methods for the treatment of heart conditions, hypertension, and other medical disorders are described. For example, this document describes devices and methods for treating atrial fibrillation by performing thoracic vein ablation procedures, including pulmonary vein myocardium ablation. In some embodiments, the ablation is performed in coordination with the delivery a pharmacological agent that can abate the formation of tissue stenosis or neointimal hyperplasia caused by the ablation. Additionally, in some embodiments, particulate matter, such as thrombus or crystalline drug compounds, created during the ablation is captured and removed from the patient using devices and methods provided herein. Further, devices and methods for non-thermal methods of causing cell death, such as tissue suction and tissue stretching, are also described.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Laser-engraveable compositions and flexographic printing precursors comprising organic porous particles
ActiveUS8613999B2Improve ablationIncrease speedLayered productsConductive materialPorous particleMaterials science
Owner:EASTMAN KODAK CO
System for direct engraving of flexographic printing members
InactiveUS8941028B2Maximize efficiencyImprove ablationAuxillary shaping apparatusMounting boardsControl layerLaser engraving
A system for engraving a flexographic relief member includes a laser scanning apparatus providing a focused radiation beam. The flexographic relief member includes a laser engraveable flexographic member; a thin engraveable control layer on top of the flexographic member; and wherein the engraveable control layer has an engraving sensitivity lower than the flexographic member.
Owner:MIRACLON CORP
Devices and methods for ablation of tissue
This document provides devices and methods for the treatment of heart conditions, hypertension, and other medical disorders. For example, this document provides devices and methods for treating atrial fibrillation by performing thoracic vein ablation procedures, including pulmonary vein myocardium ablation. In some embodiments, the ablation is performed in coordination with the delivery a pharmacological agent that can abate the formation of tissue stenosis or neointimal hyperplasia caused by the ablation.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com