Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Homocystinuria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Homocystinuria (HCU) is a rare but potentially serious inherited condition.
Glycine betaine and its use
InactiveUS7608640B2Reduce adhesive and migration propertyReduce aggressionSalicyclic acid active ingredientsBiocideGlycineBetaine
Owner:MESSADEK JALLAL
Novel choline cocrystal of epalrestat
The invention relates to a novel choline cocrystal of 5-[(lZ.2E)-2-methyl-3-phenylpropenylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid. The preparation and characterization of the novel choline cocrystal according to various embodiments of the invention is described. The invention also relates to pharmaceutical compositions containing the novel choline cocrystal and the therapeutic use of the novel choline cocrystal to treat and / or prevent various conditions, including treating and / or preventing diabetic complications, treating and / or preventing homocystinuria reducing levels of homocysteine in blood serum, inhibiting aldose reductase, and affording cardioprotection in non-diabetic patients.
Owner:BIONEVIA PHARMACEUTICALS INC
Chemically modified cystathionine beta-synthase enzyme for treatment of homocystinuria
ActiveUS9034318B2Reducing serum HcyHigh activityOrganic active ingredientsSenses disorderMedicineCystinuria
The invention provides reagents and methods for enzyme replacement therapy using chemically modified species of human cystathionine β-synthase (CBS) to treat homocystinuria and other related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
Compositions and methods for treatment of homocystinuria
Provided herein are improved compositions and methods for enzyme replacement therapy using modified human cystathionine beta synthase (CBS) in the treatment of homocystinuria and related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
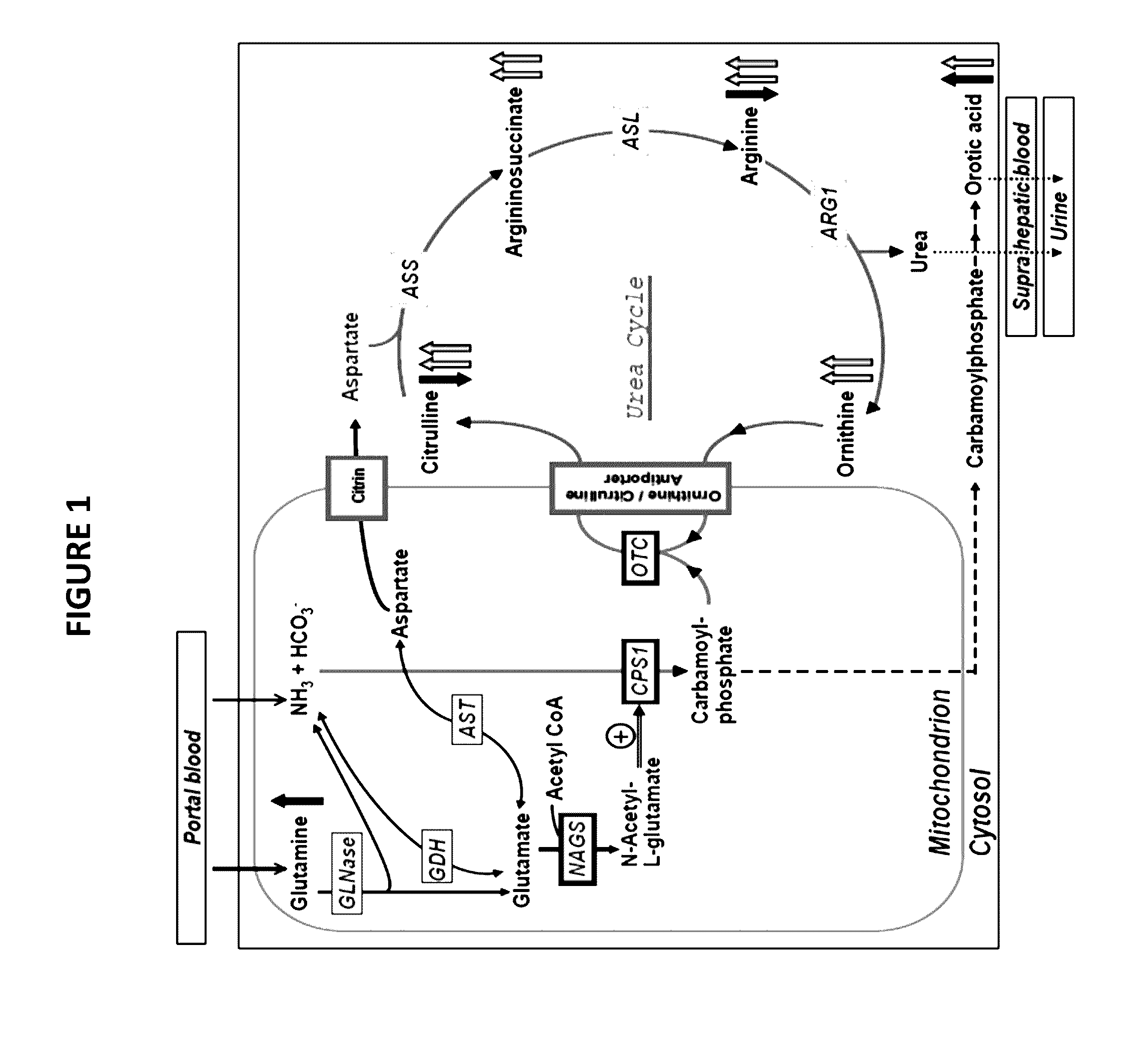
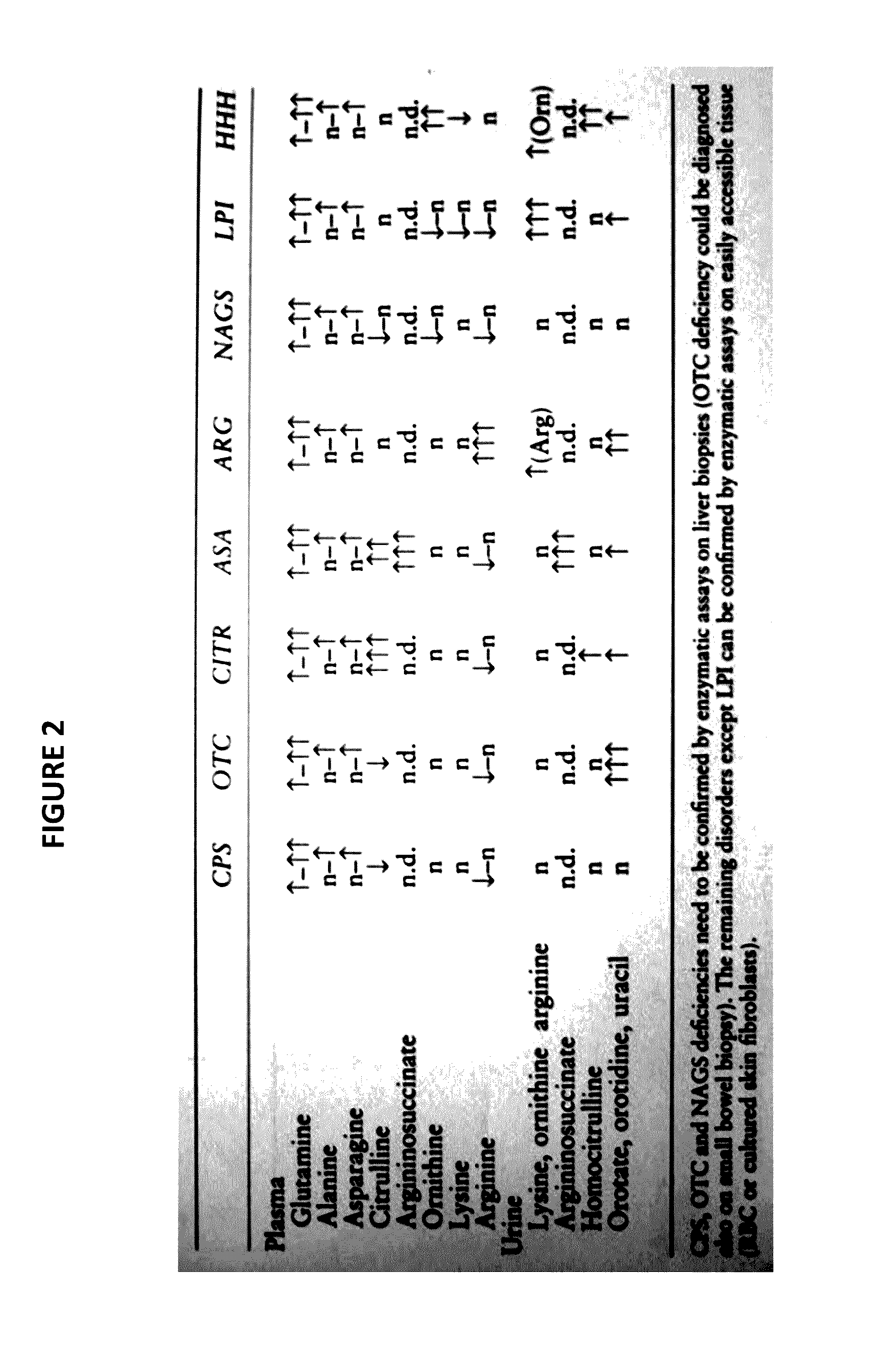
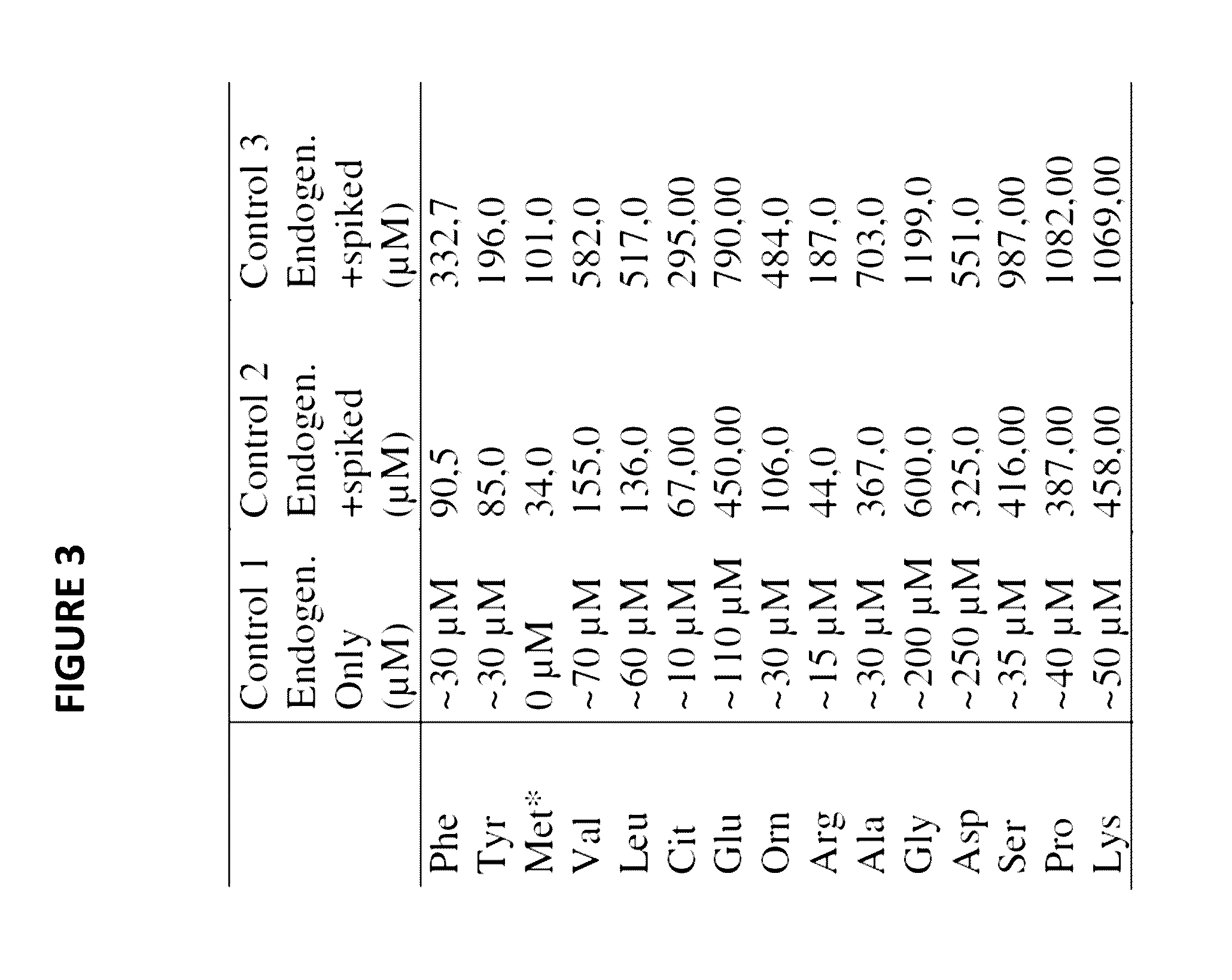
Novel methods and kits for detecting of urea cycle disorders using mass spectrometry
InactiveUS20170059535A1Component separationBiological testingStable Isotope LabelingHigh-Throughput Screening Methods
The present invention relates to newborn screening kits, methods, stable isotopically-labeled internal standards or internal standard solution for high throughput screening and analysis of metabolic disorders using liquid chromatography mass spectrometry (LC-MS) are provided. The metabolic disorders can be amino acid, organic acid or fatty acid oxidation disorders, and particularly urea cycle disorders or deficiencies, hyperammonemia, Hyperornithinemia-hyperammonemia-homocitrullinuria (HHH), and / or argininosuccinic aciduria. The newborn screening kits, methods, stable isotopically-labeled internal standards or internal standard solution are particularly useful for newborn screening (NBS) of metabolic disorders.
Owner:LABSYST DIAGNOSTICS OY
Compositions and methods for treatment of homocystinuria
Provided herein are improved compositions and methods for enzyme replacement therapy using modified human cystathionine beta synthase (CBS) in the treatment of homocystinuria and related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
Therapeutic cell systems and methods for treating homocystinuria
InactiveUS20190309271A1Reduced immune reactionLower Level RequirementsCarbon-sulfur lyasesPeptide/protein ingredientsCysteine degradationRed blood cell
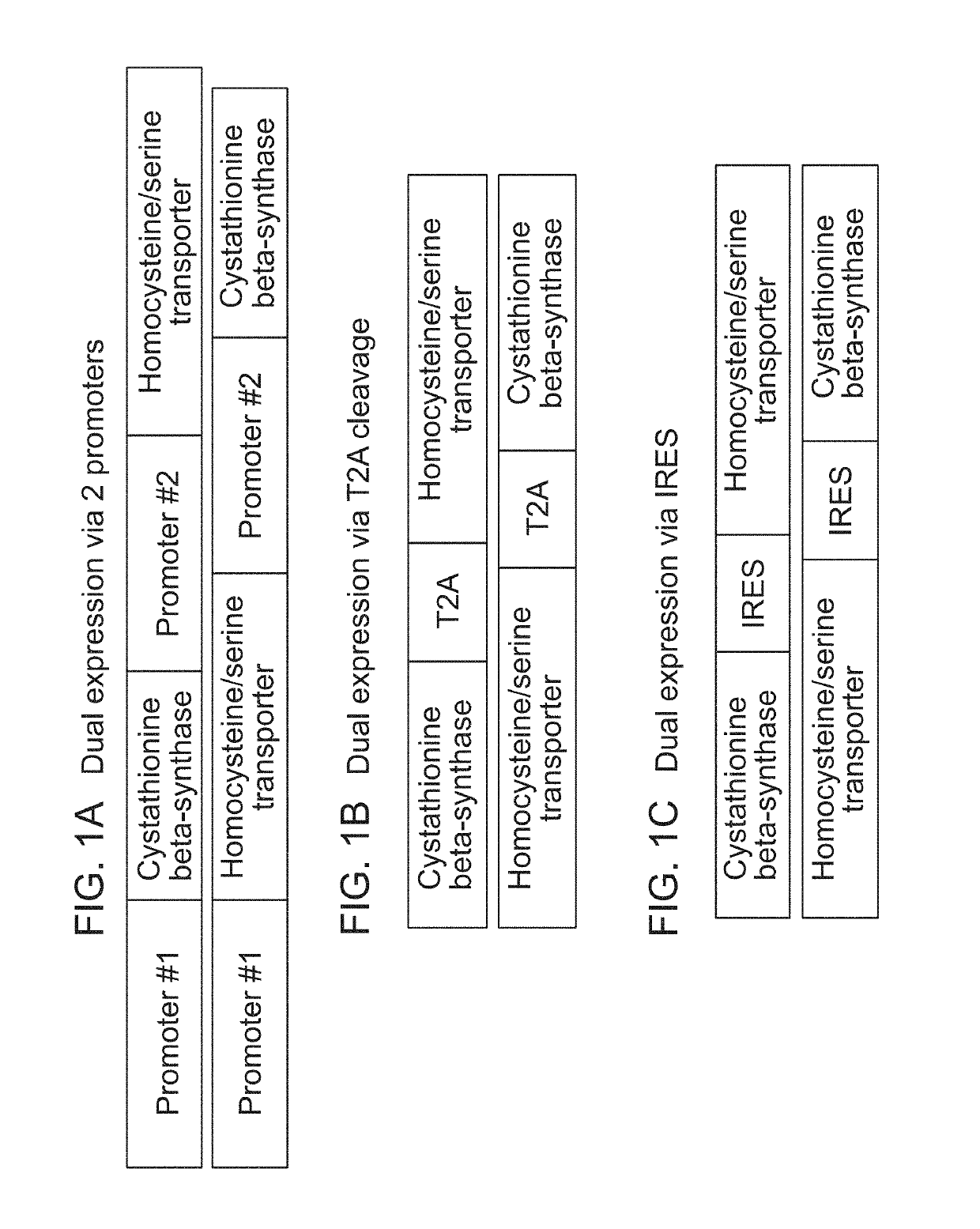
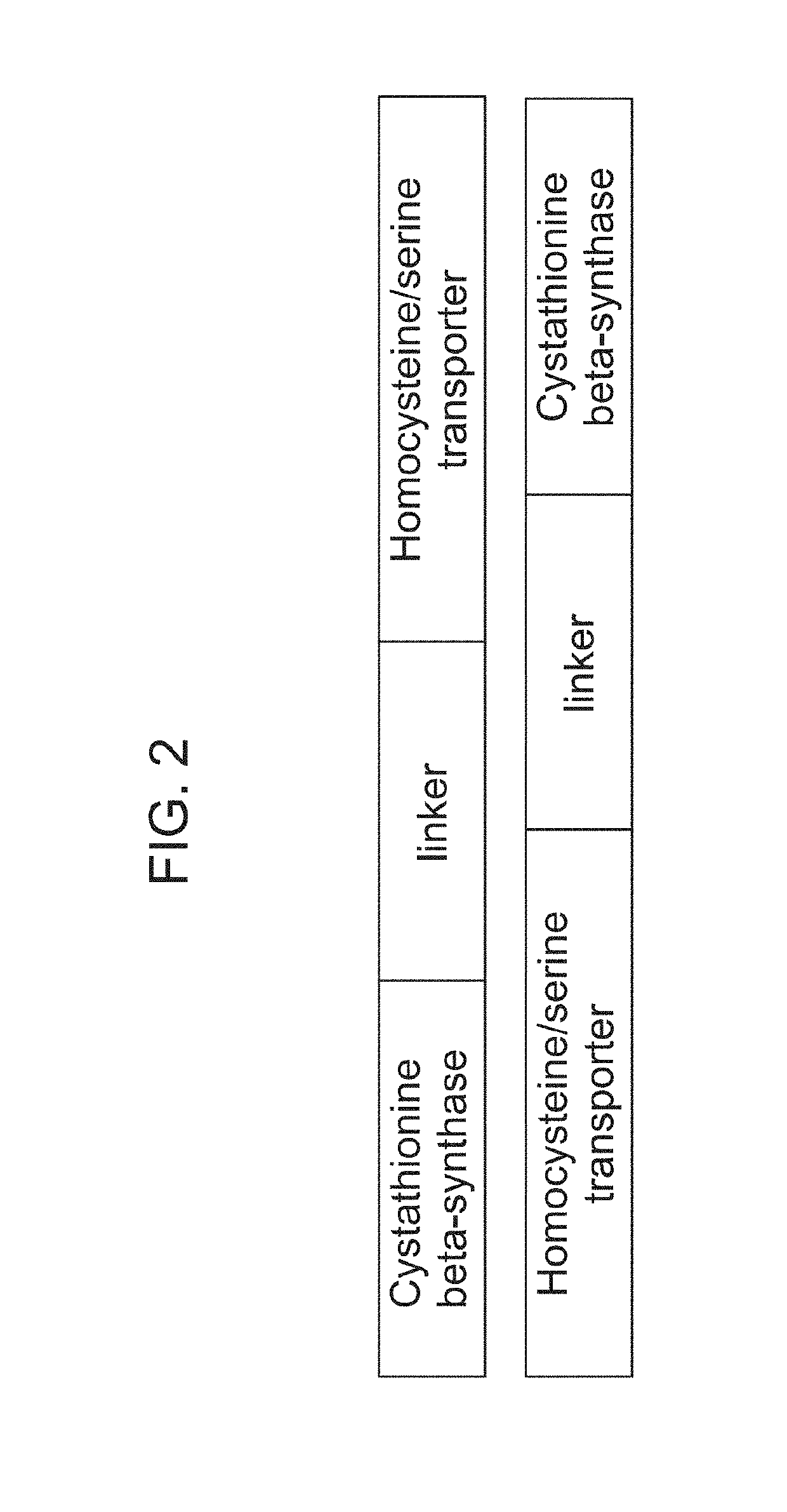
The present disclosure relates to erythroid cells that have been engineered to express a homocysteine reducing polypeptide, or a variant thereof, or a homocysteine degrading polypeptide, or a variant thereof. The engineered erythroid cells may further comprise an amino acid transporter, for example a homocysteine transporter or a serine transporter, or a cystathionine degrading polypeptide. The engineered erythroid cells of the present disclosure are useful in reducing the level of homocysteine in a subject. The engineered erythroid cells of the present disclosure are further useful in methods of treating homocystinuria.
Owner:RUBIUS THERAPEUTICS
Purification of multi-specific receptors
Disclosed is a method for preparing a composition enriched for receptors (typically molecular impringet polymers, MIPs) that bind an agent, where said receptors each specifically bind at least two discrete sites on said agent, by subjecting a sample of receptors to a first step of affinity purification with the agent where one binding site on the agent is non-accessible for binding to the receptors and subsequently subjecting the purified receptors to at least one further step of affinity purification with the agent where a second binding site on the agent is non-accessible. Also disclosed is a method for treatment, amelioration or prophylaxis of a disease selected from the group consisting of phenylketonuria (PKU, Følling's disease), hyperphenylalaninemia (HPA), alcaptonuria (black urine disease), tyrosinemia, hypertyrosinemia, myasthenia gravis, histidinemia, urocanic aciduria, maple syrup urine disease (MSUD), isovaleric acidemia (isovaleryl-CoA dehydrogenase deficiency), homocystinuria, propionic acidemia, methylmalonic acidemia, and glutaric aciduria Type 1 (GA-I), galactosemia, comprising administering to the gastrointestinal tract of a patient in need thereof an effective amount of a composition of molecular imprinted polymers (MIPs), said composition being capable of binding a symptom provoking agent of said disease.
Owner:MIPSALUS APS
Human-enzyme mediated depletion of homocysteine for treating patients with hyperhomocysteinemia and homocystinuria
ActiveUS20180326025A1Reduce homocysteine levelEfficient degradationOrganic active ingredientsCarbon-sulfur lyasesAmino acid substitutionHyperhomocysteinemia
Methods and compositions relating to the engineering of an improved protein with homocyst(e)inase enzyme activity are described. For example, there are disclosed modified cystathionine-γ-lyase (CGL) enzymes comprising one or more amino acid substitutions and capable of degrading homocyst(e)ine. Furthermore, provided are compositions and methods for the treatment of homocystinuria or hyperhomocysteinemia with homocyst(e)ine depletion using the disclosed enzymes or nucleic acids.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Glycine betaine and its use
InactiveUS20100004199A1Reduce expressionReduce adhesive and migration propertyBiocideOrganic active ingredientsGlycineBetaine
Owner:MESSADEK JALLAL
Novel choline cocrystal of epalrestat
The invention relates to a novel choline cocrystal of 5-[(1Z,2E)-2-methyl-3-phenylpropenylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid. The preparation and characterization of the novel choline cocrystal according to various embodiments of the invention is described. The invention also relates to pharmaceutical compositions containing the novel choline cocrystal and the therapeutic use of the novel choline cocrystal to treat and / or prevent various conditions, including treating and / or preventing diabetic complications, treating and / or preventing homocystinuria reducing levels of homocysteine in blood serum, inhibiting aldose reductase, and affording cardioprotection in non-diabetic patients.
Owner:BIONEVIA PHARMACEUTICALS INC
Chemically modified cystathionine beta-synthase enzyme for treatment of homocystinuria
ActiveUS20140212403A1Reducing serum HcyImprove stabilityOrganic active ingredientsSenses disorderMedicineCystinuria
The invention provides reagents and methods for enzyme replacement therapy using chemically modified species of human cystathionine β-synthase (CBS) to treat homocystinuria and other related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
Glycine betaine and its use
InactiveUS20130005683A1Reduce expressionReduce adhesive and migration propertyOrganic active ingredientsBiocideGlycineBetaine
Owner:MESSADEK JALLAL
Purification of multi-specific receptors
Disclosed is a method for preparing a composition enriched for receptors (typically molecular impringet polymers, MIPs) that bind an agent, where said receptors each specifically bind at least two discrete sites on said agent, by subjecting a sample of receptors to a first step of affinity purification with the agent where one binding site on the agent is non-accessible for binding to the receptors and subsequently subjecting the purified receptors to at least one further step of affinity purification with the agent where a second binding site on the agent is non-accessible. Also disclosed is a method for treatment, amelioration or prophylaxis of a disease selected from the group consisting of phenylketonuria (PKU, Følling's disease), hyperphenylalaninemia (HPA), alcaptonuria (black urine disease), tyrosinemia, hypertyrosinemia, myasthenia gravis, histidinemia, urocanic aciduria, maple syrup urine disease (MSUD), isovaleric acidemia (isovaleryl-CoA dehydrogenase deficiency), homocystinuria, propionic acidemia, methylmalonic acidemia, and glutaric aciduria Type 1 (GA-I), galactosemia, comprising administering to the gastrointestinal tract of a patient in need thereof an effective amount of a composition of molecular imprinted polymers (MIPs), said composition being capable of binding a symptom provoking agent of said disease.
Owner:MIPSALUS APS
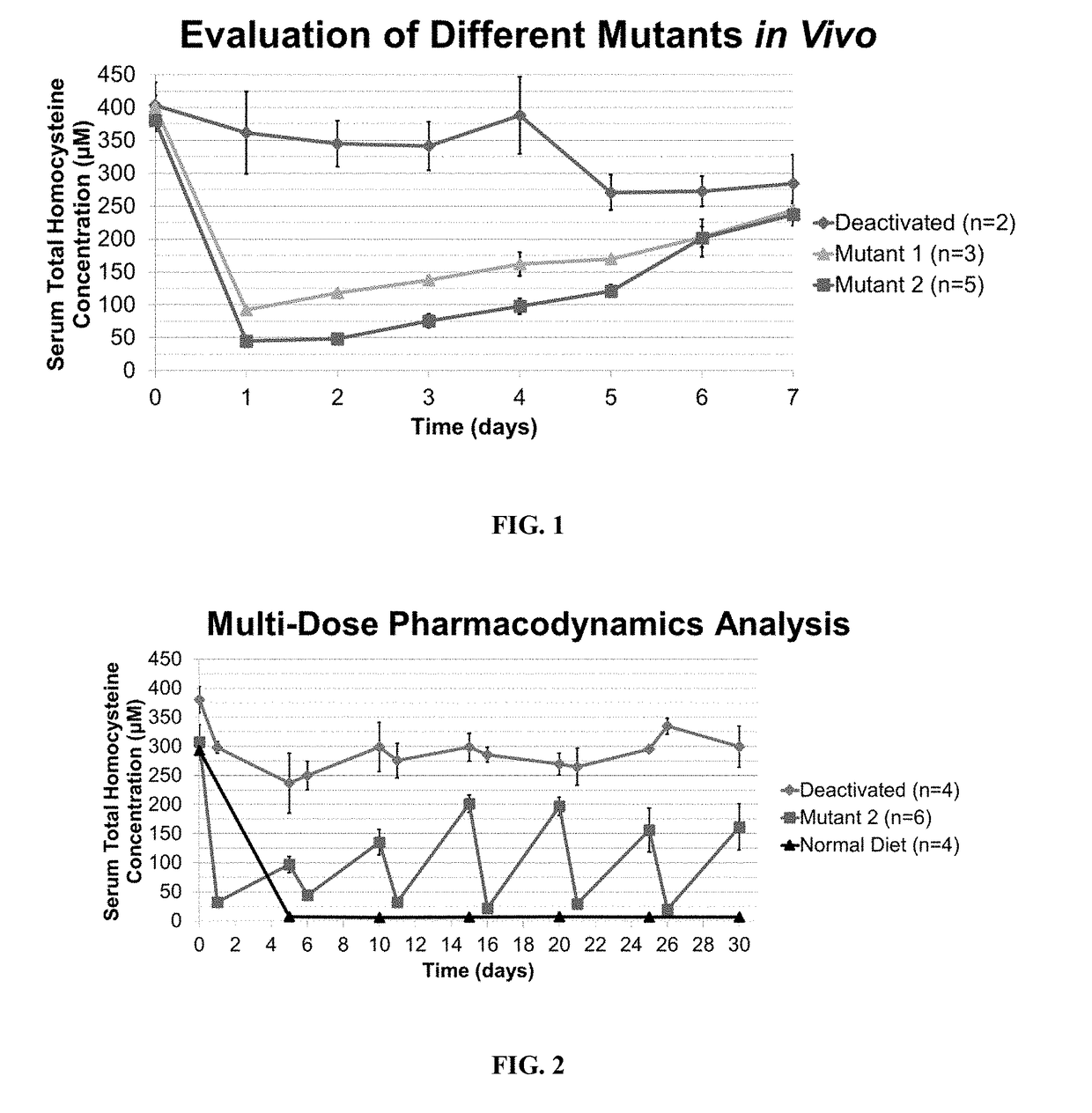
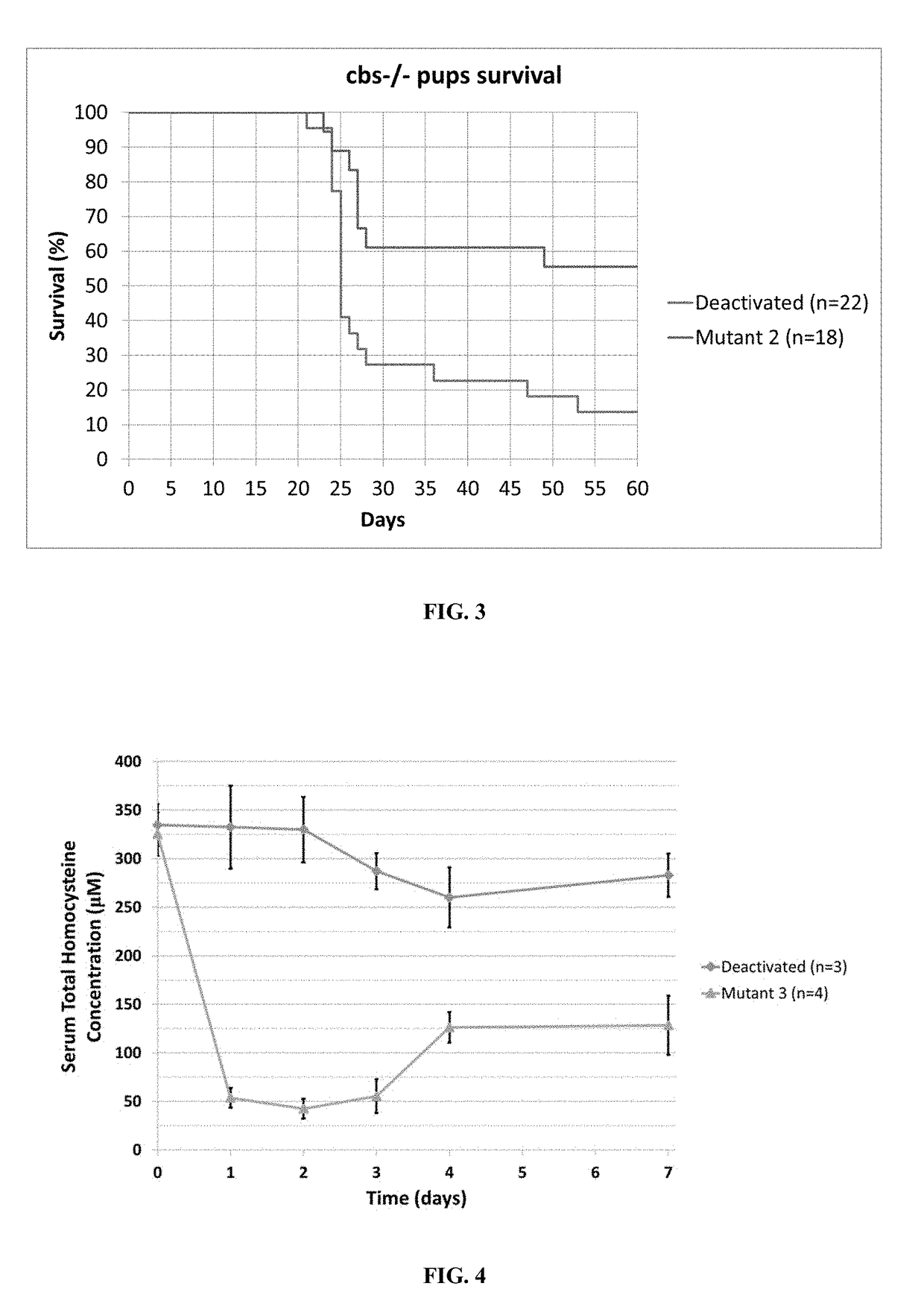
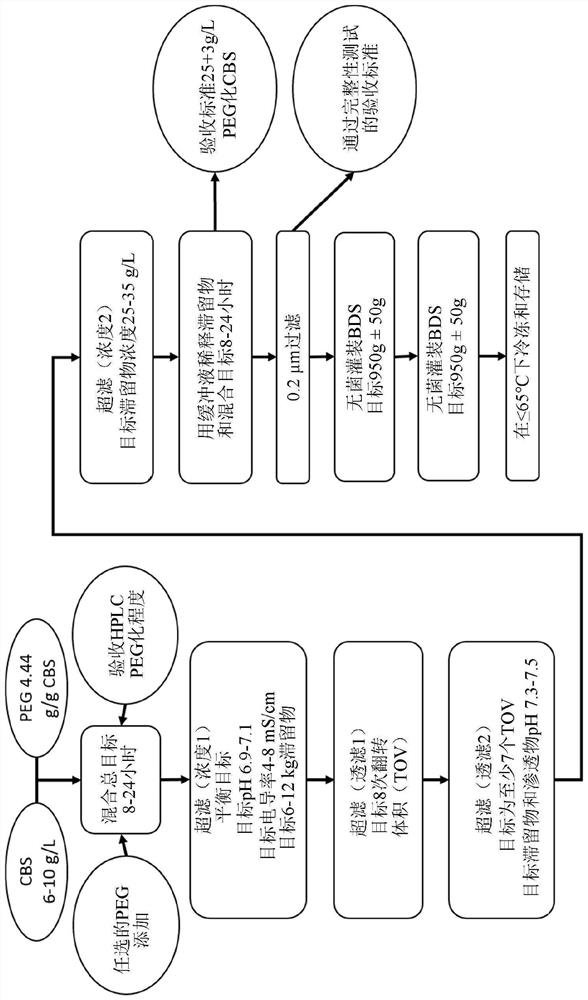
Optimization of enzyme replacement therapy for treatment of homocystinuria
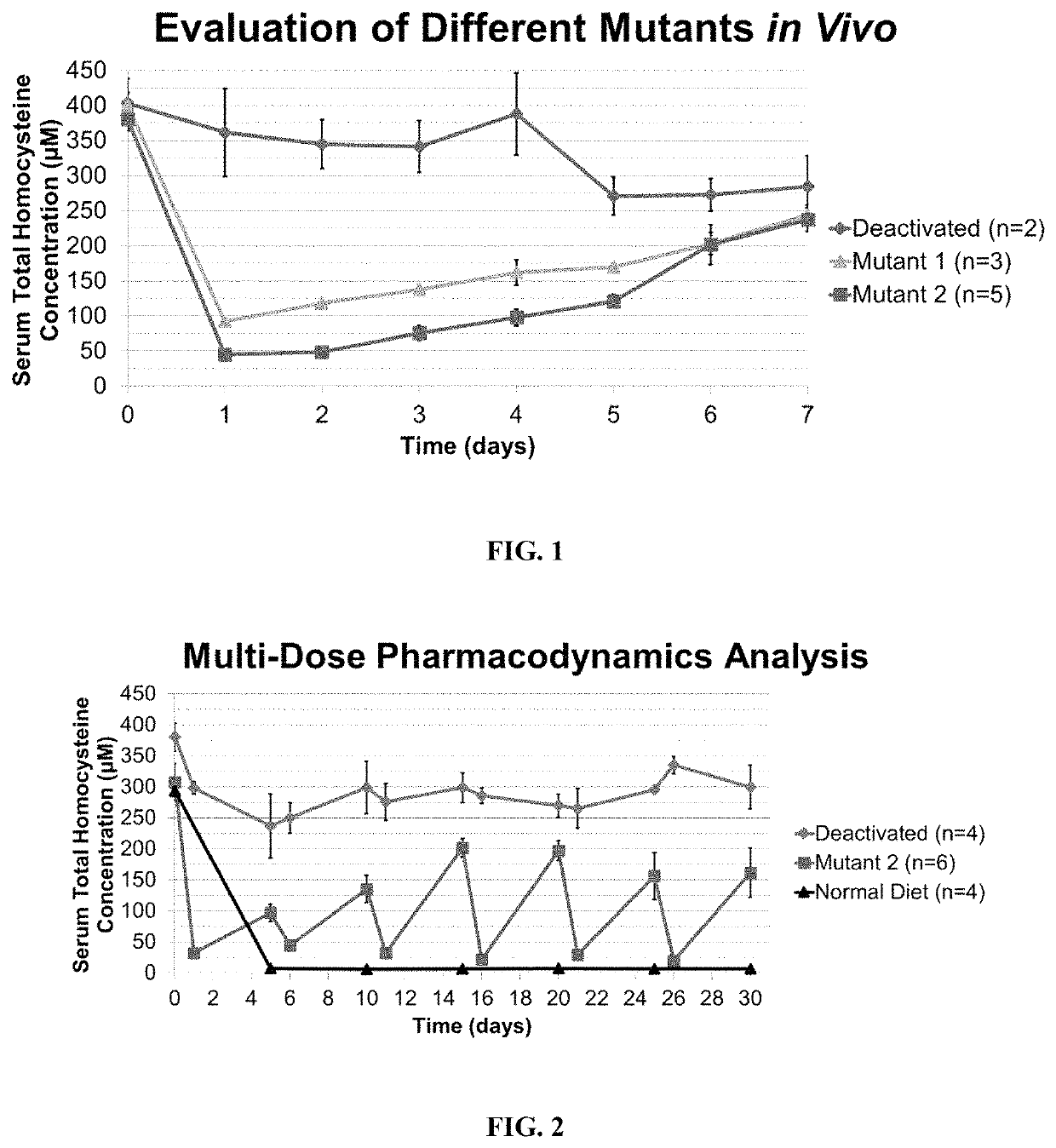
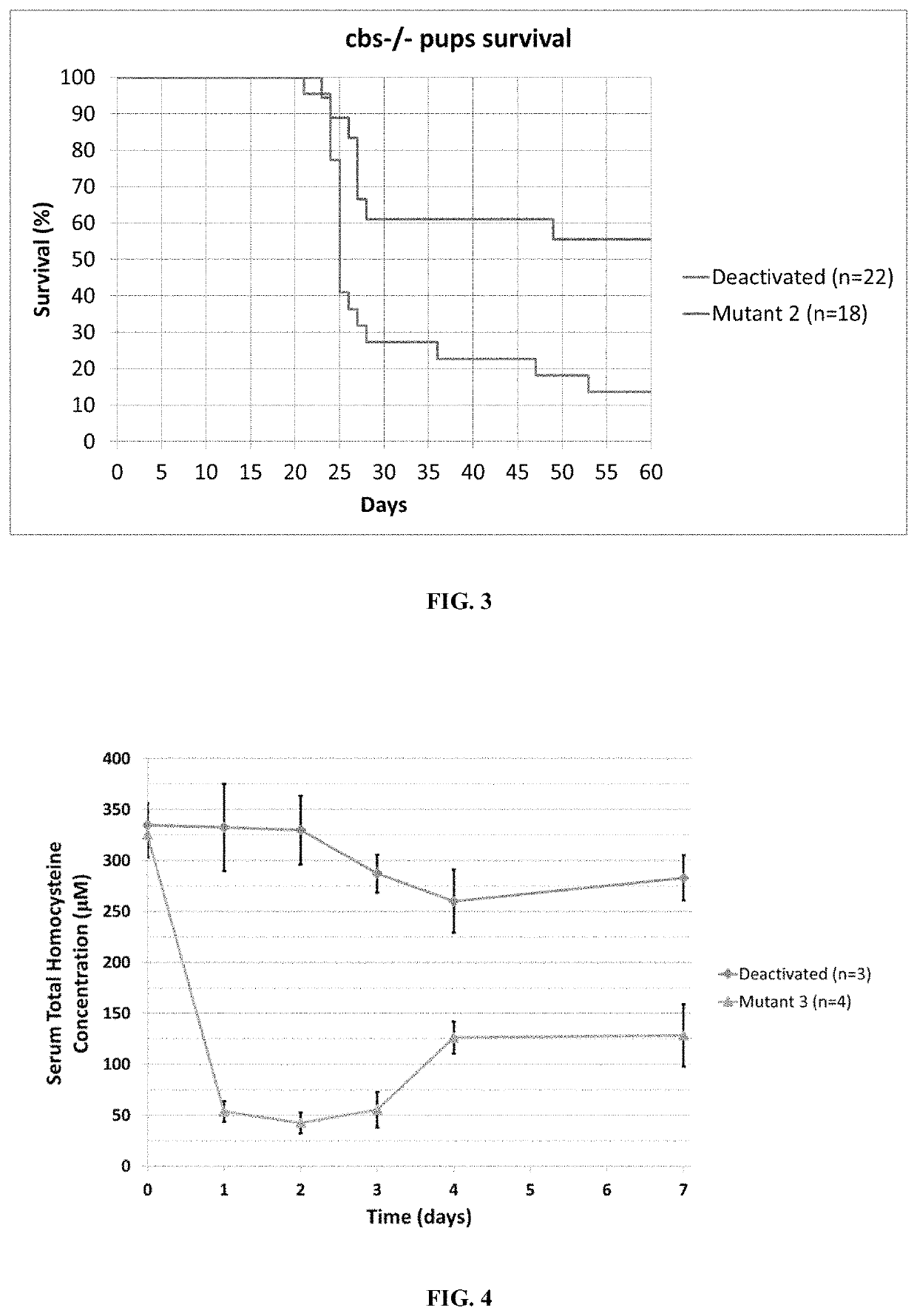
ActiveUS11324811B2Reduce the amount requiredDecrease in levelPeptide/protein ingredientsEnzyme stabilisationEnzyme proteinHomocystine
Owner:UNIV OF COLORADO THE REGENTS OF +1
Optimization of enzyme replacement therapy for treatment of homocystinuria
ActiveUS20200261555A1Reduce the amount requiredDecrease in levelPeptide/protein ingredientsEnzyme stabilisationEnzyme proteinHomocystine
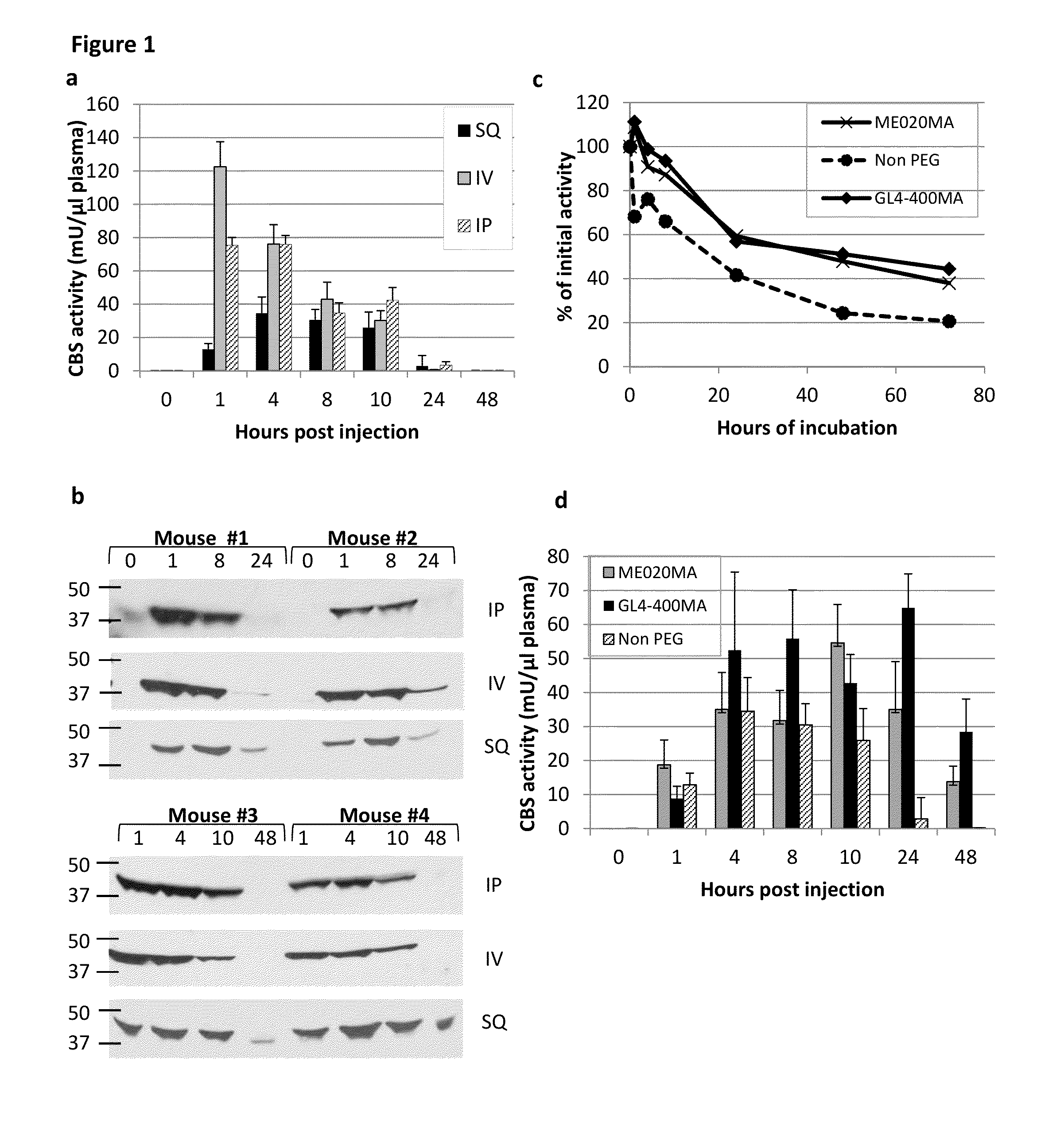
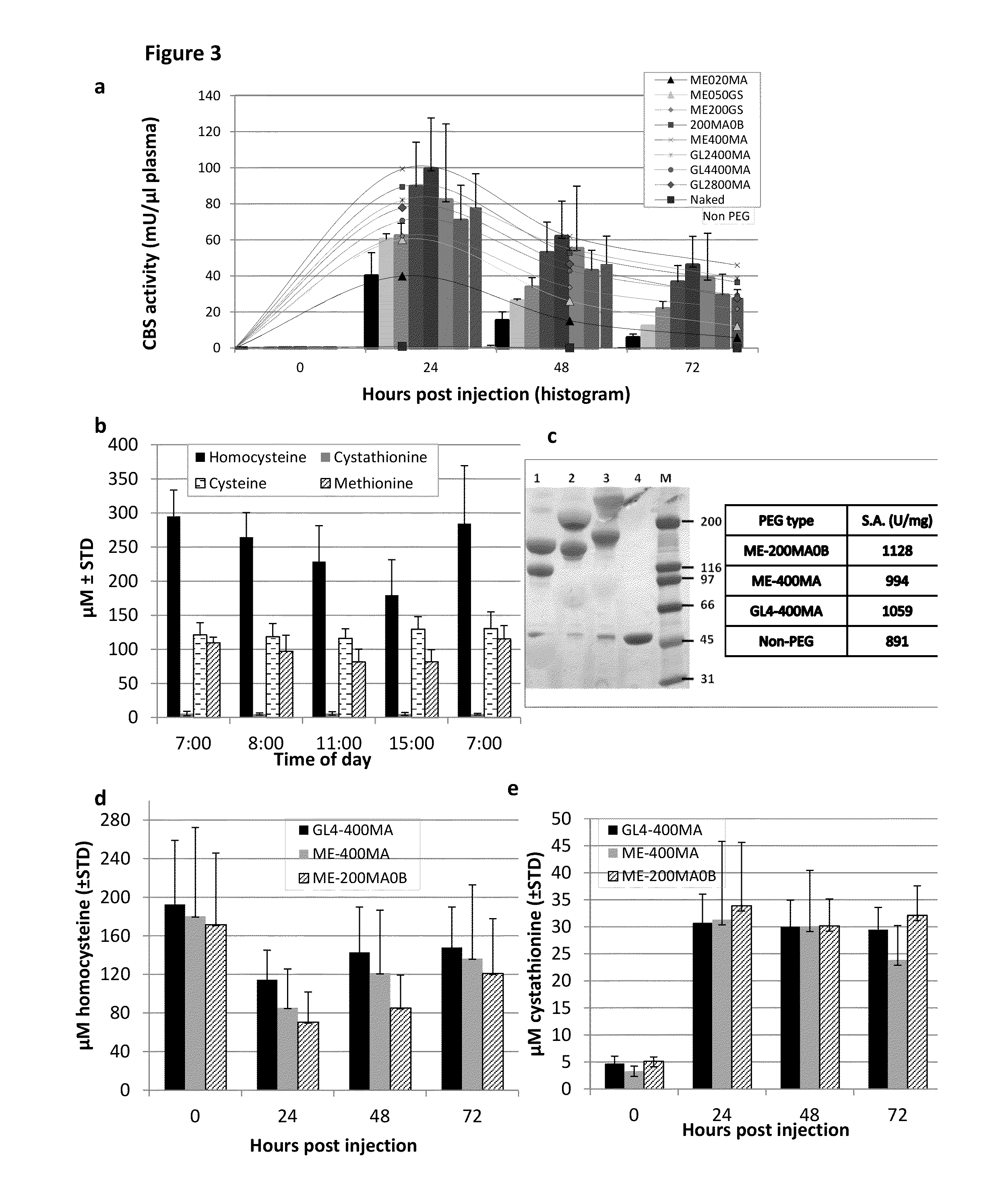
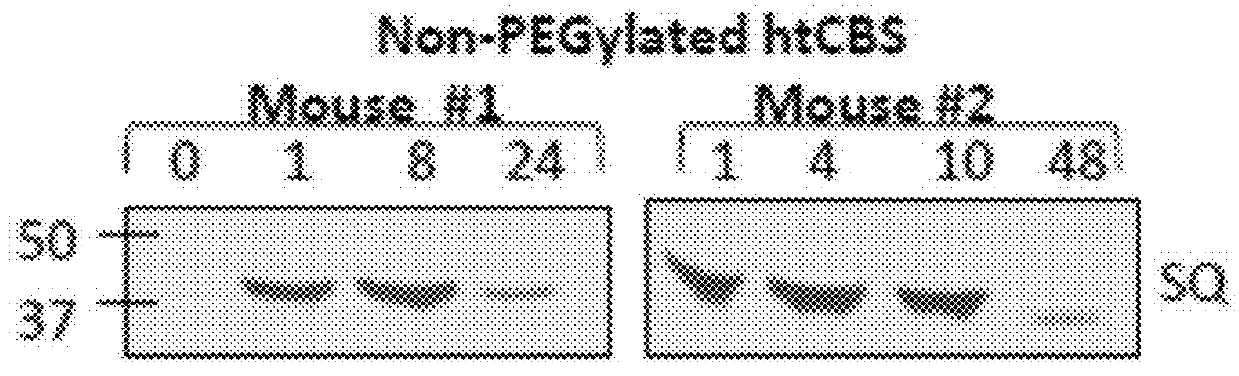
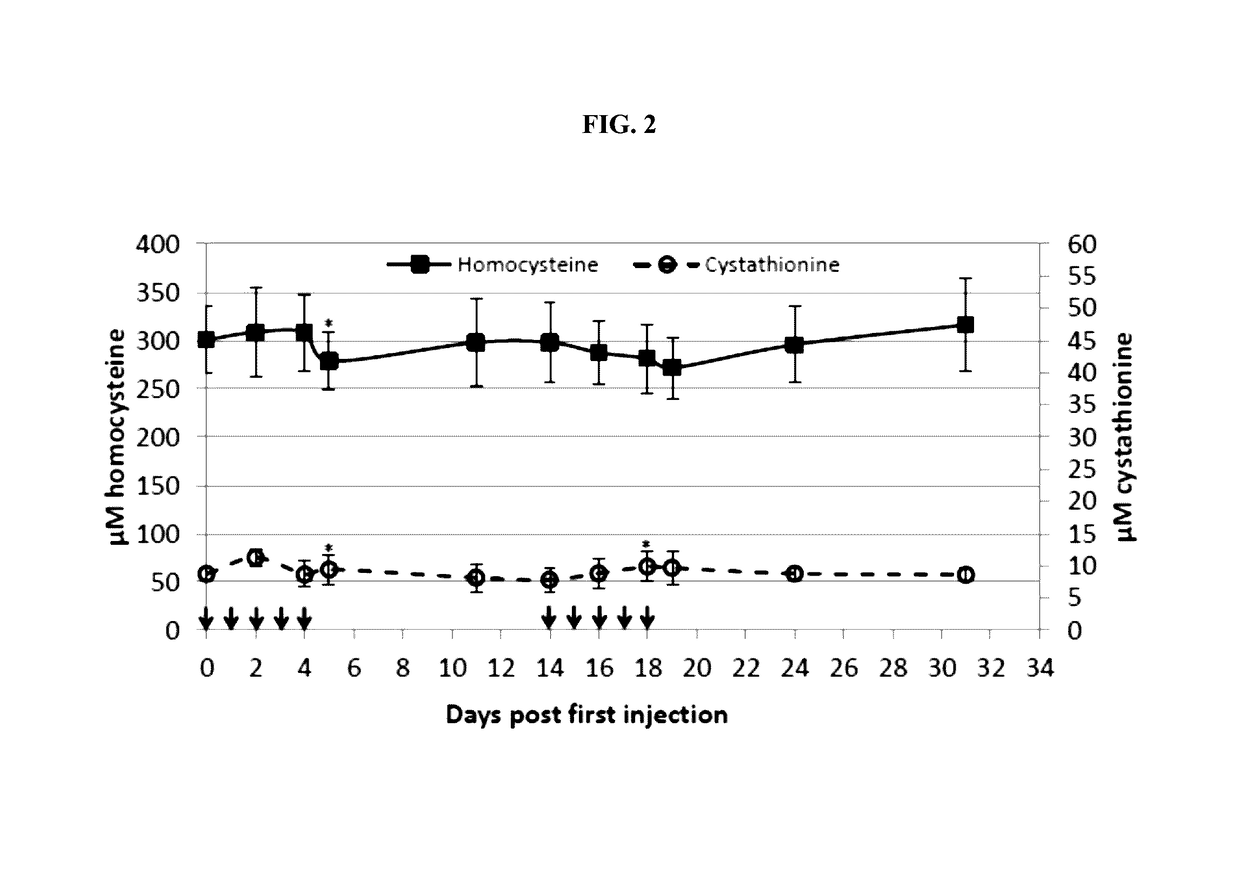
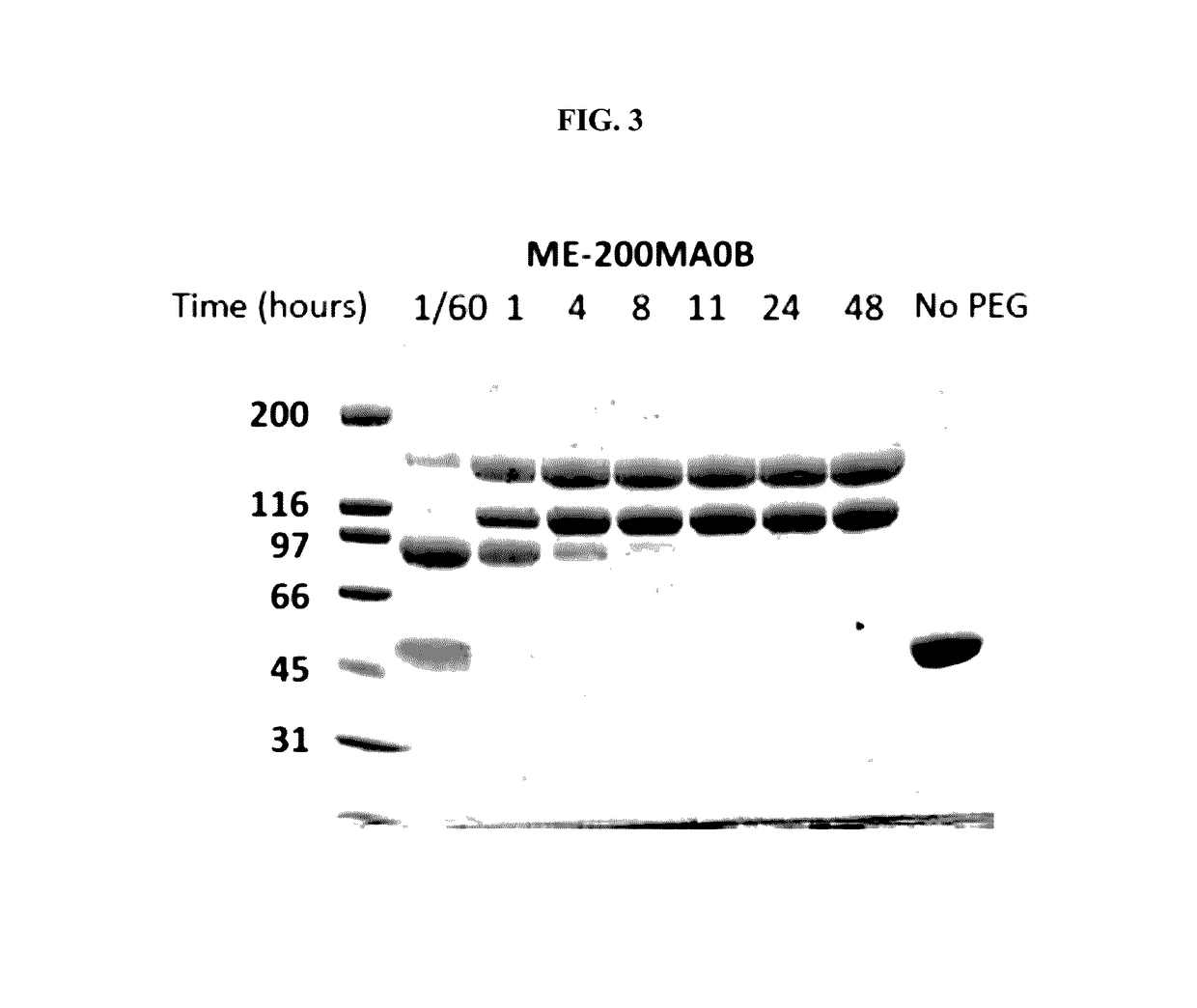
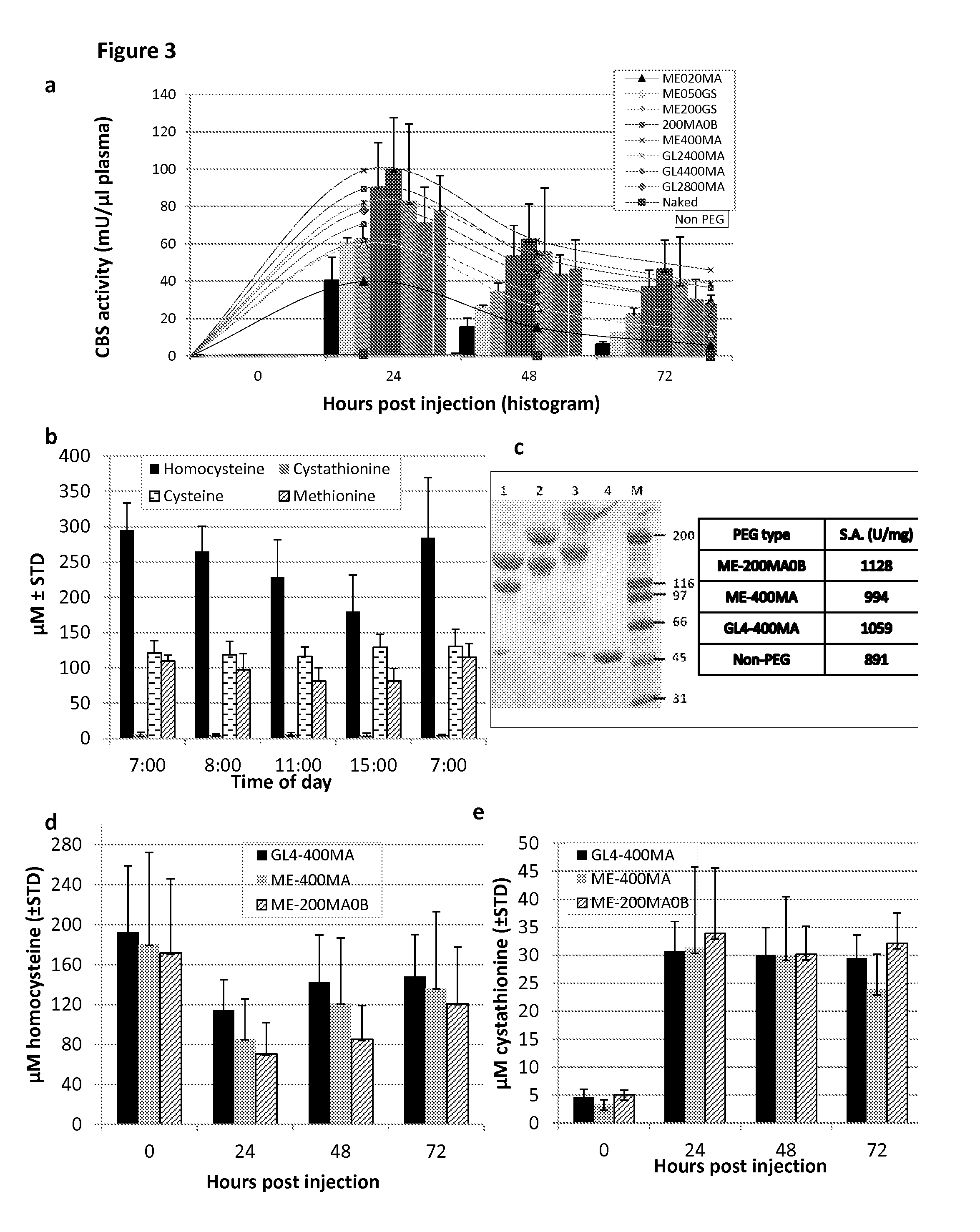
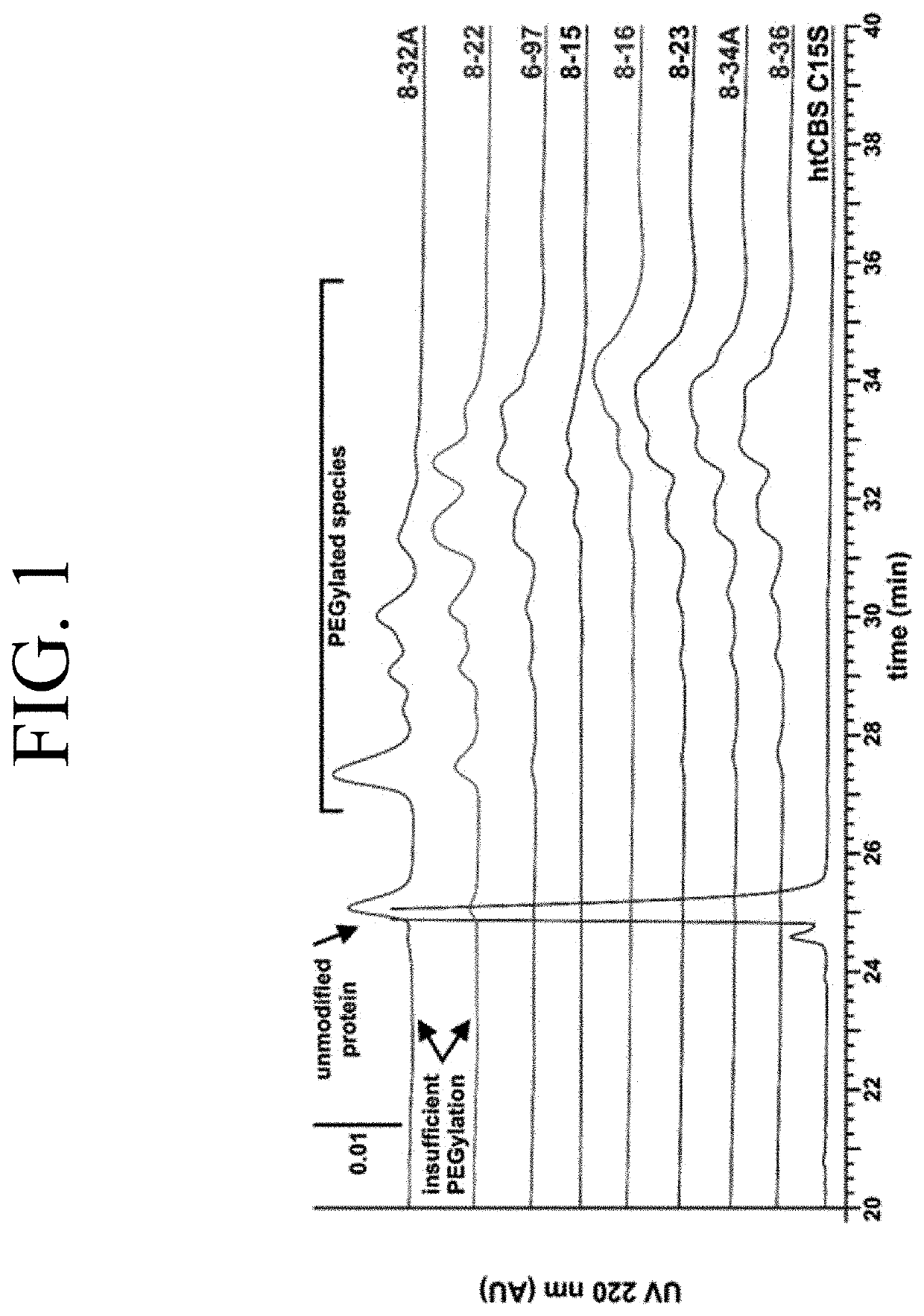
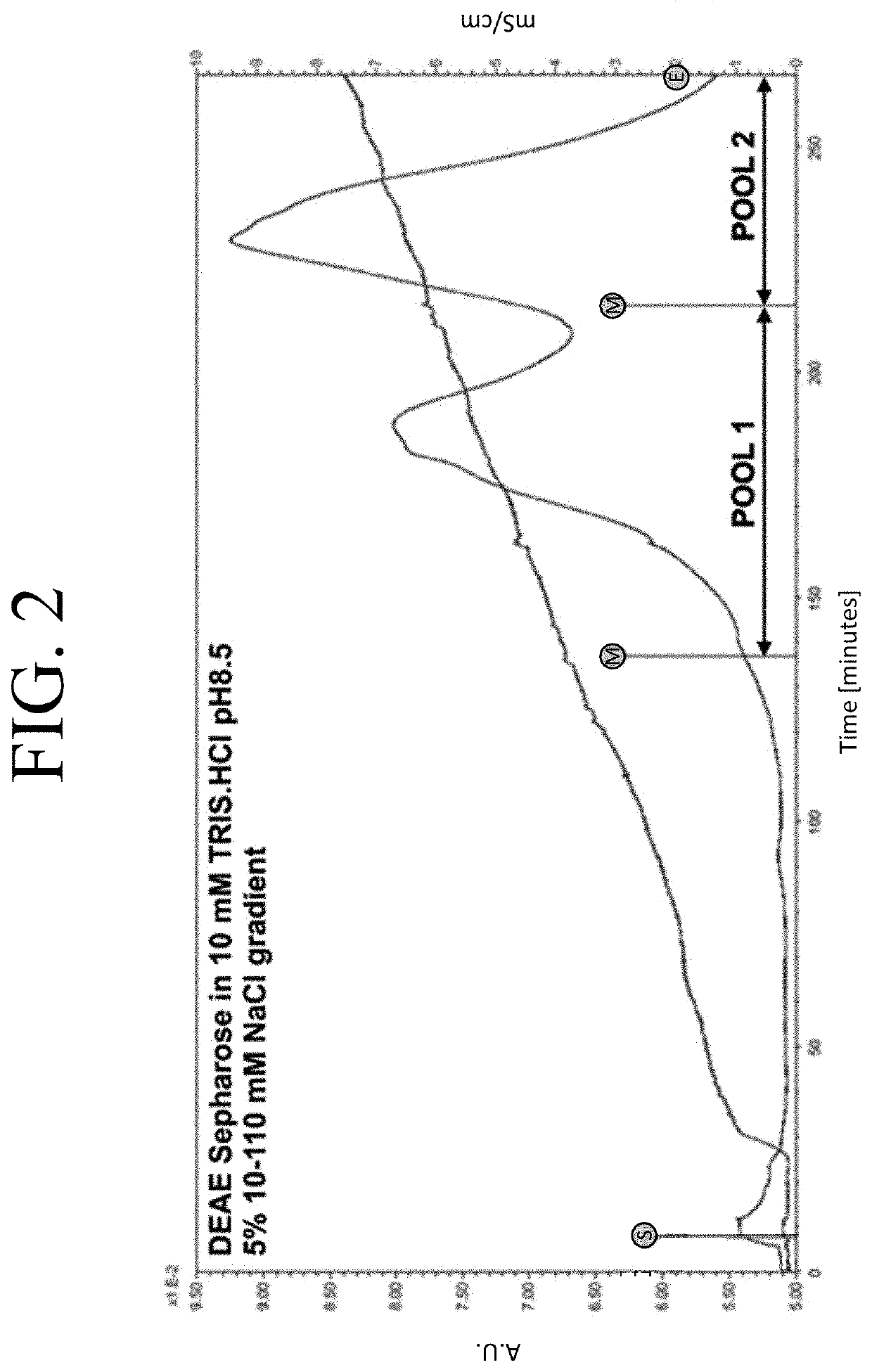
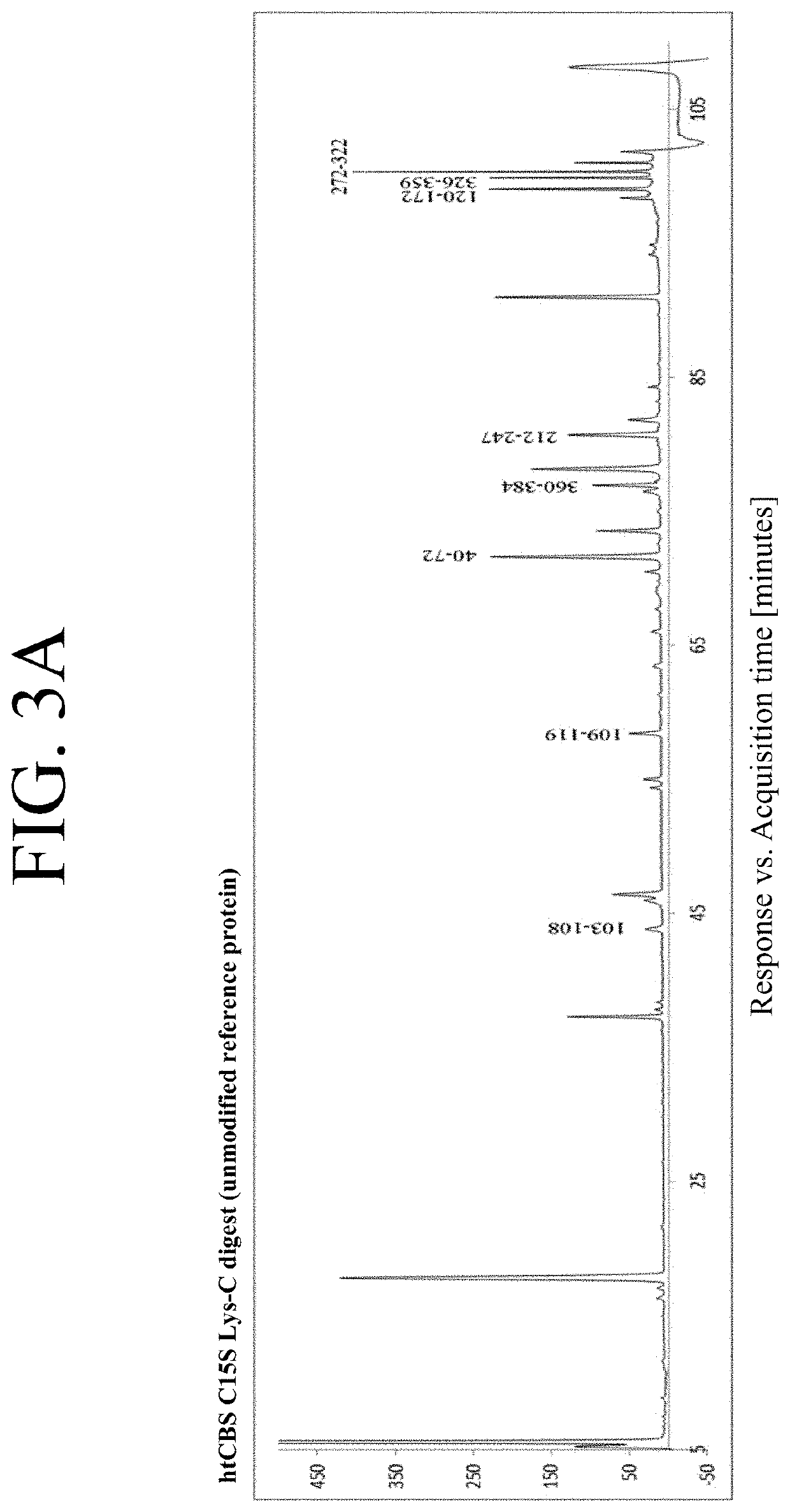
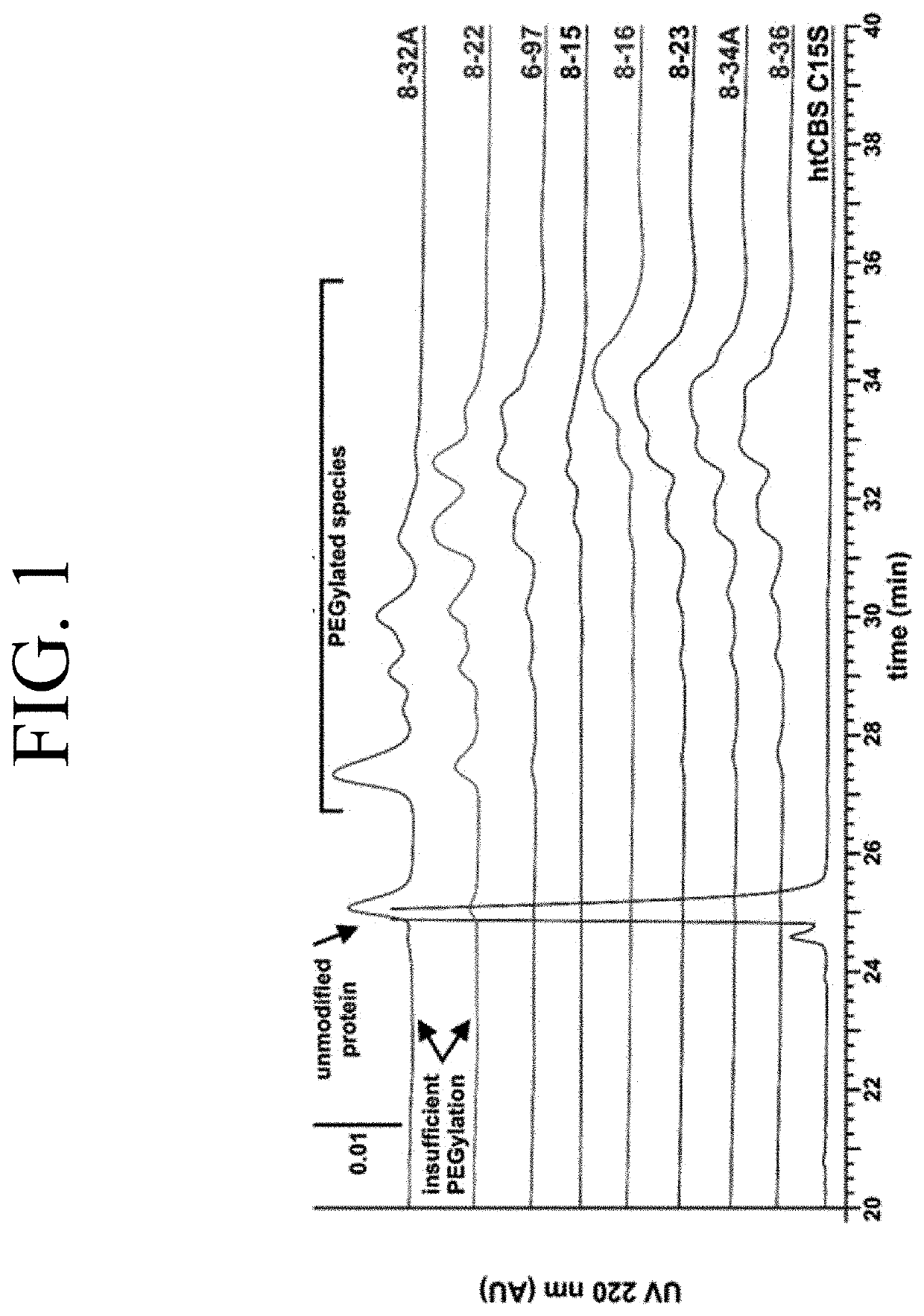
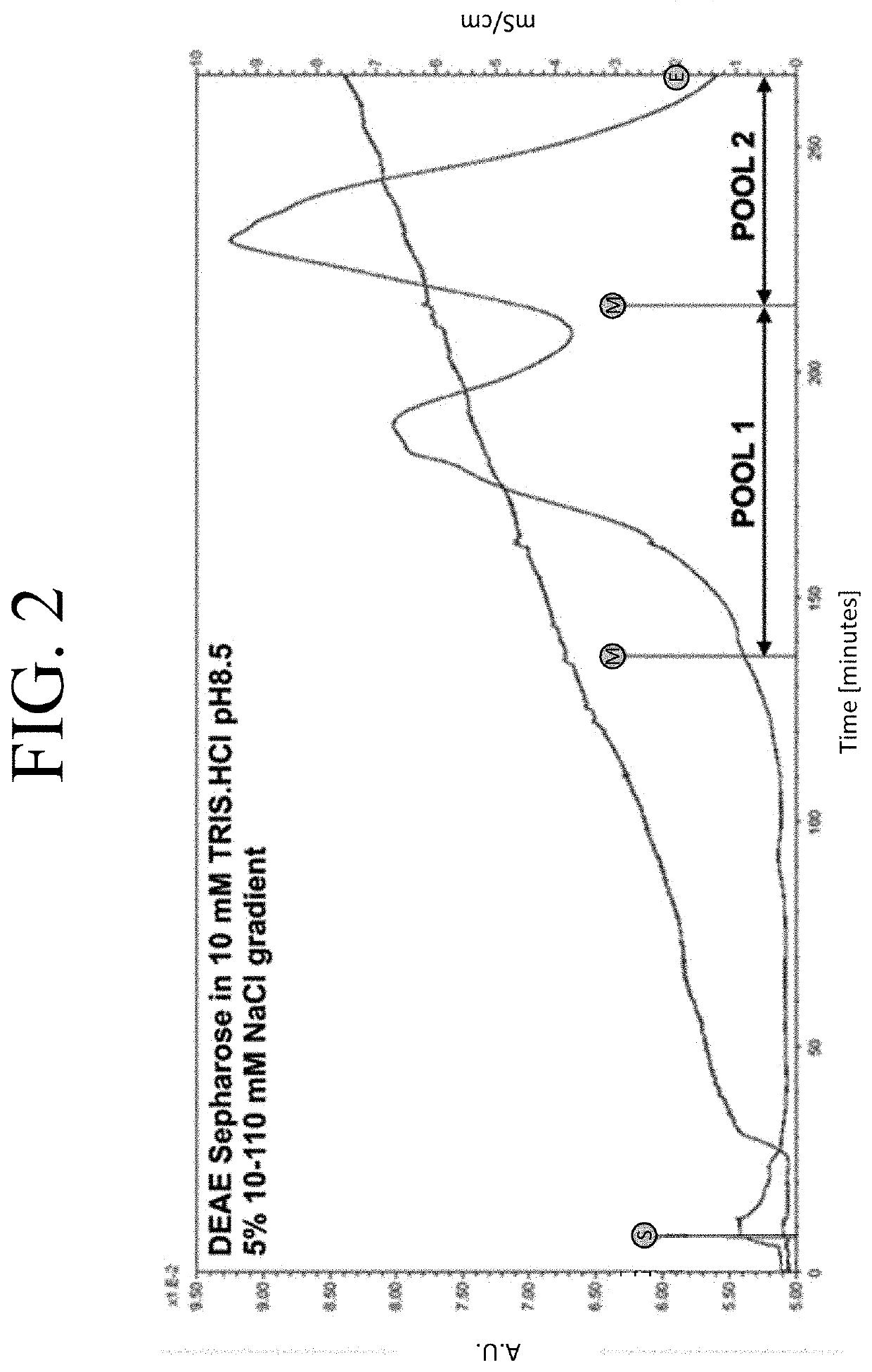
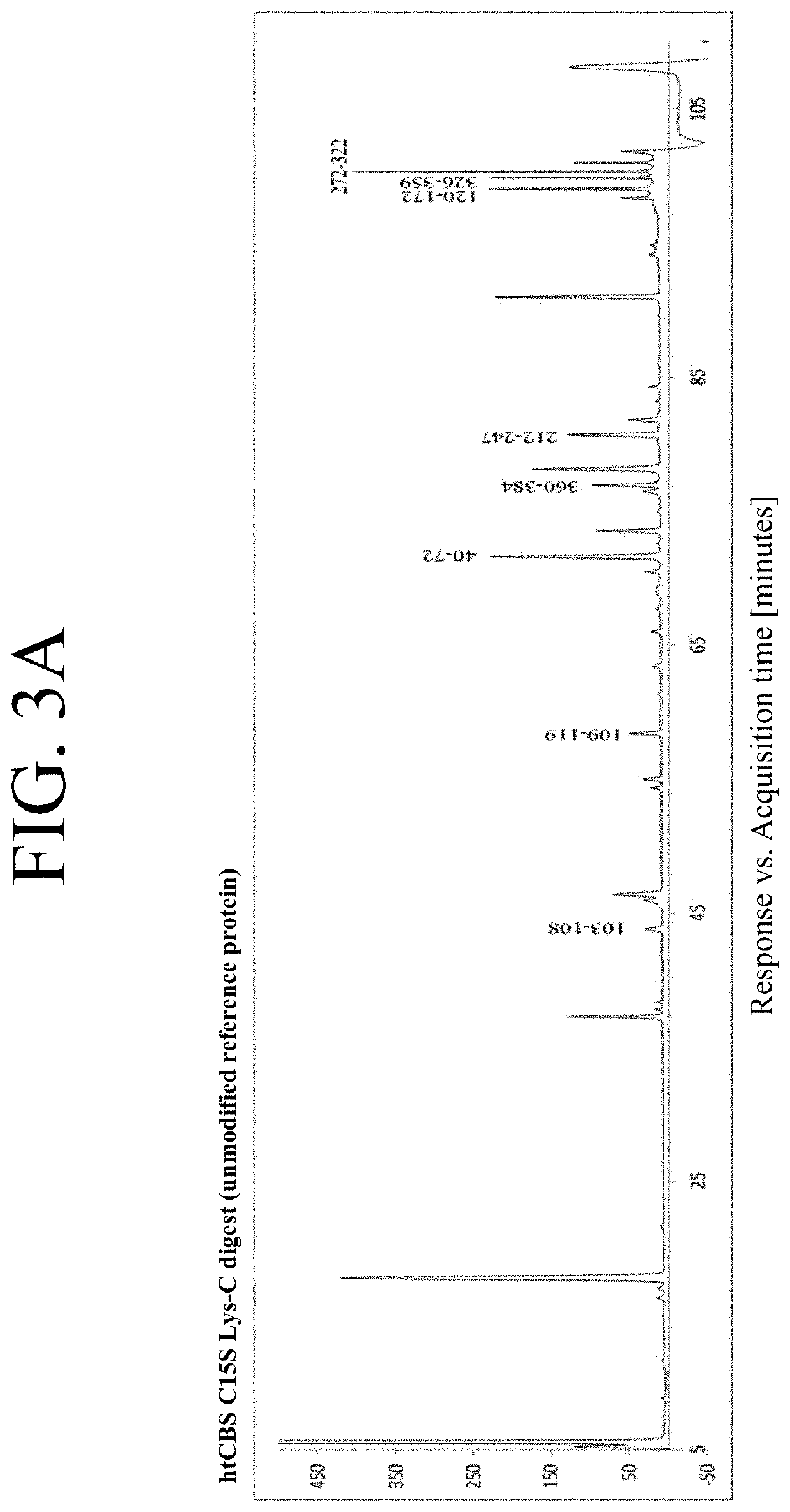
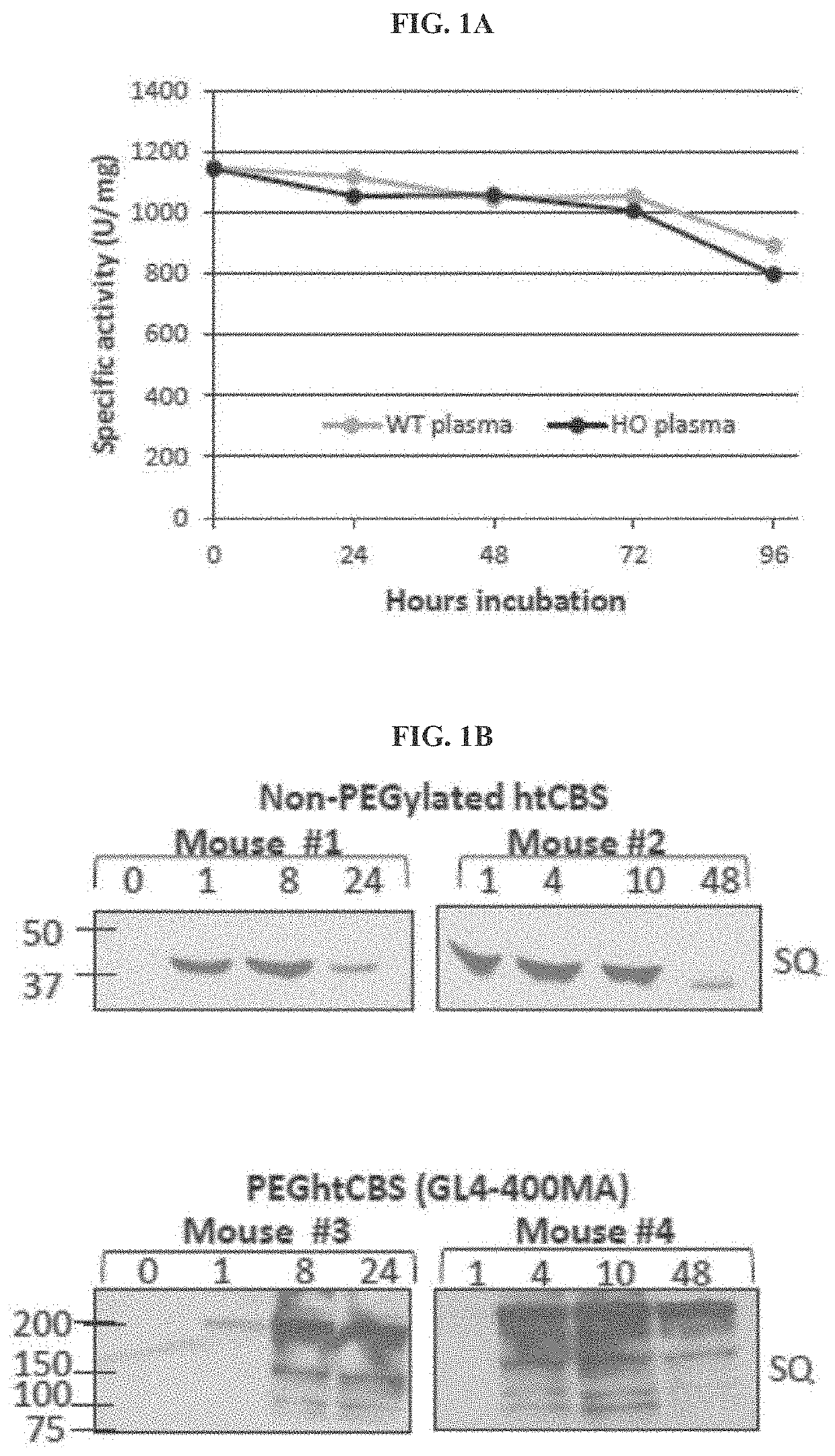
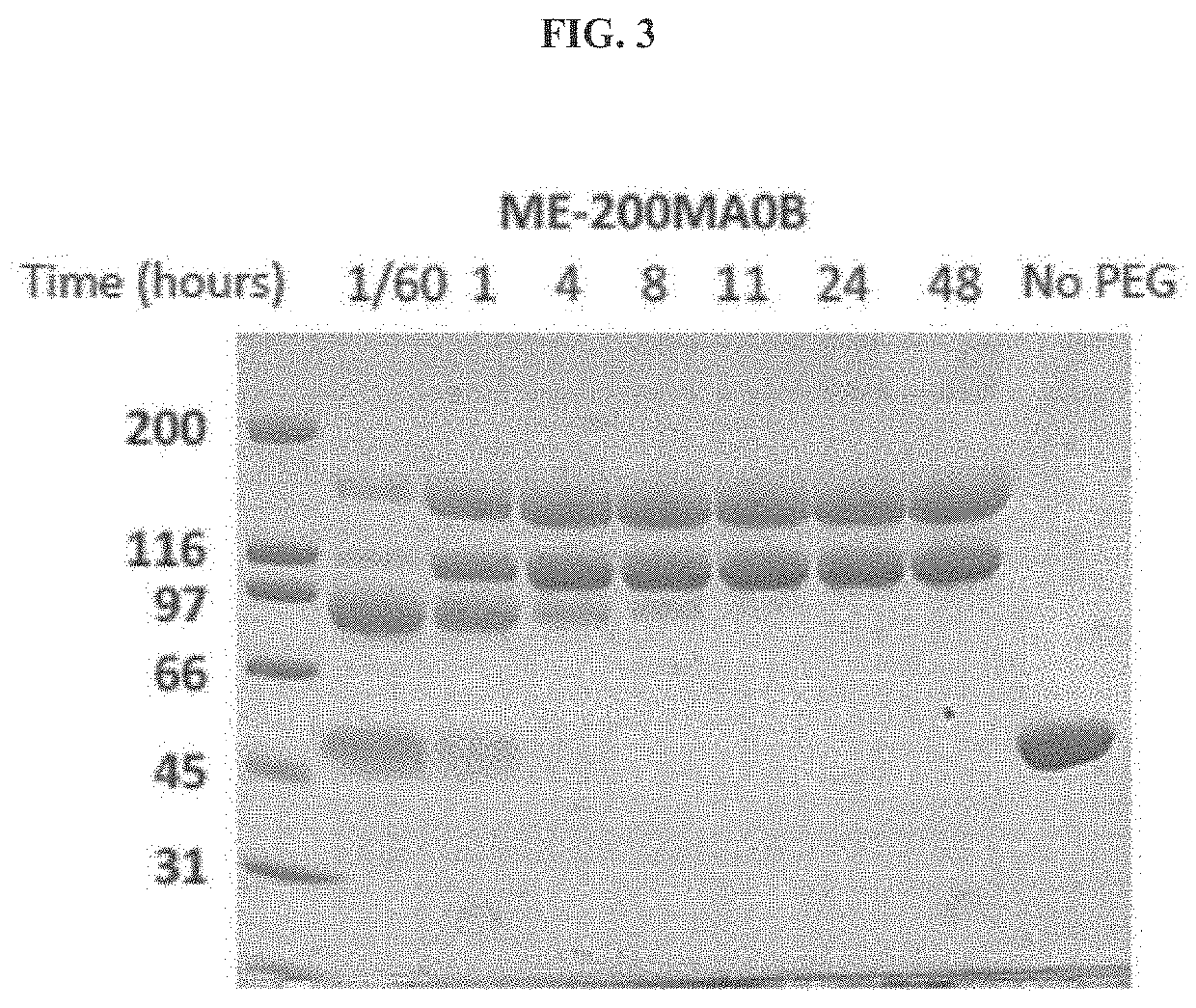
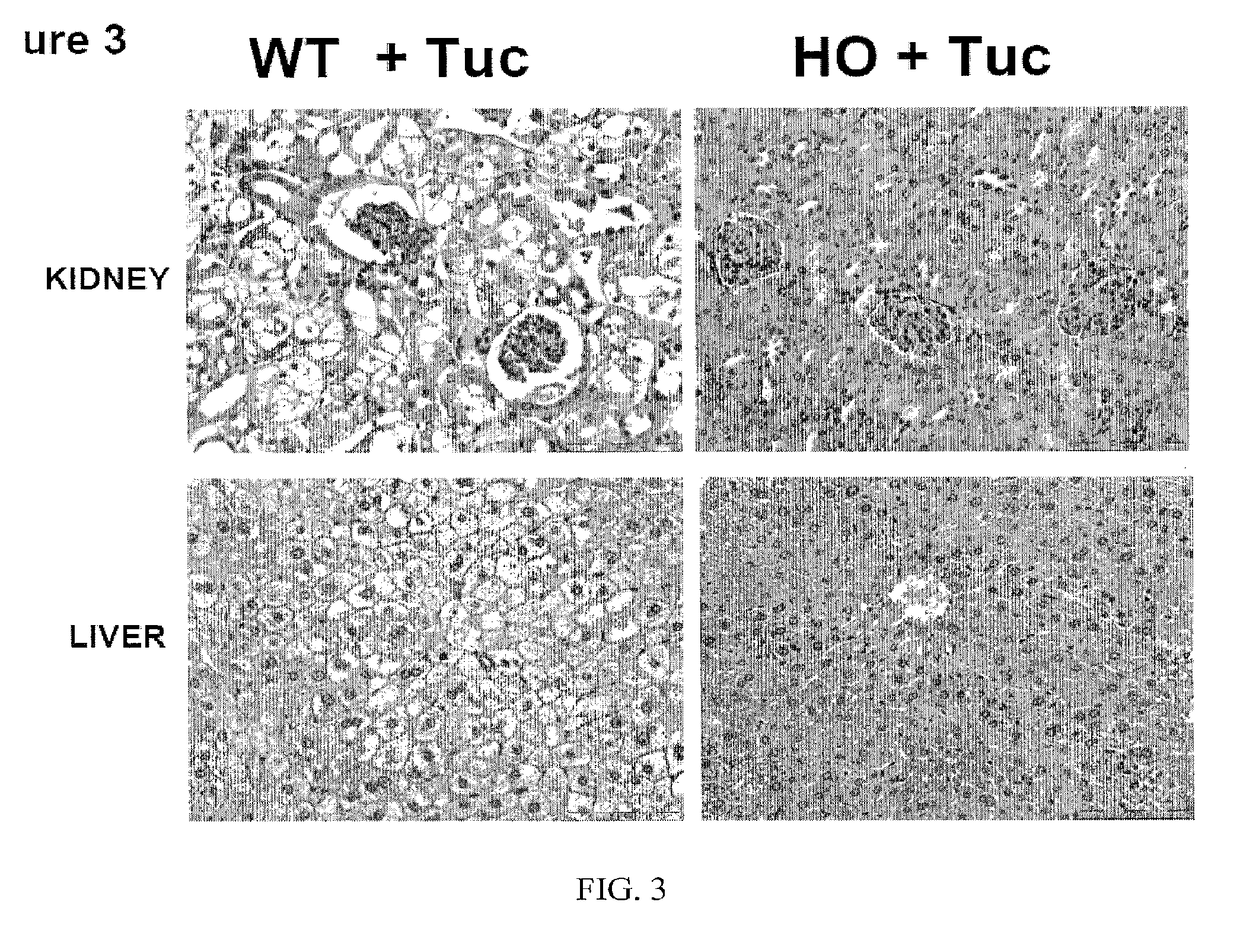
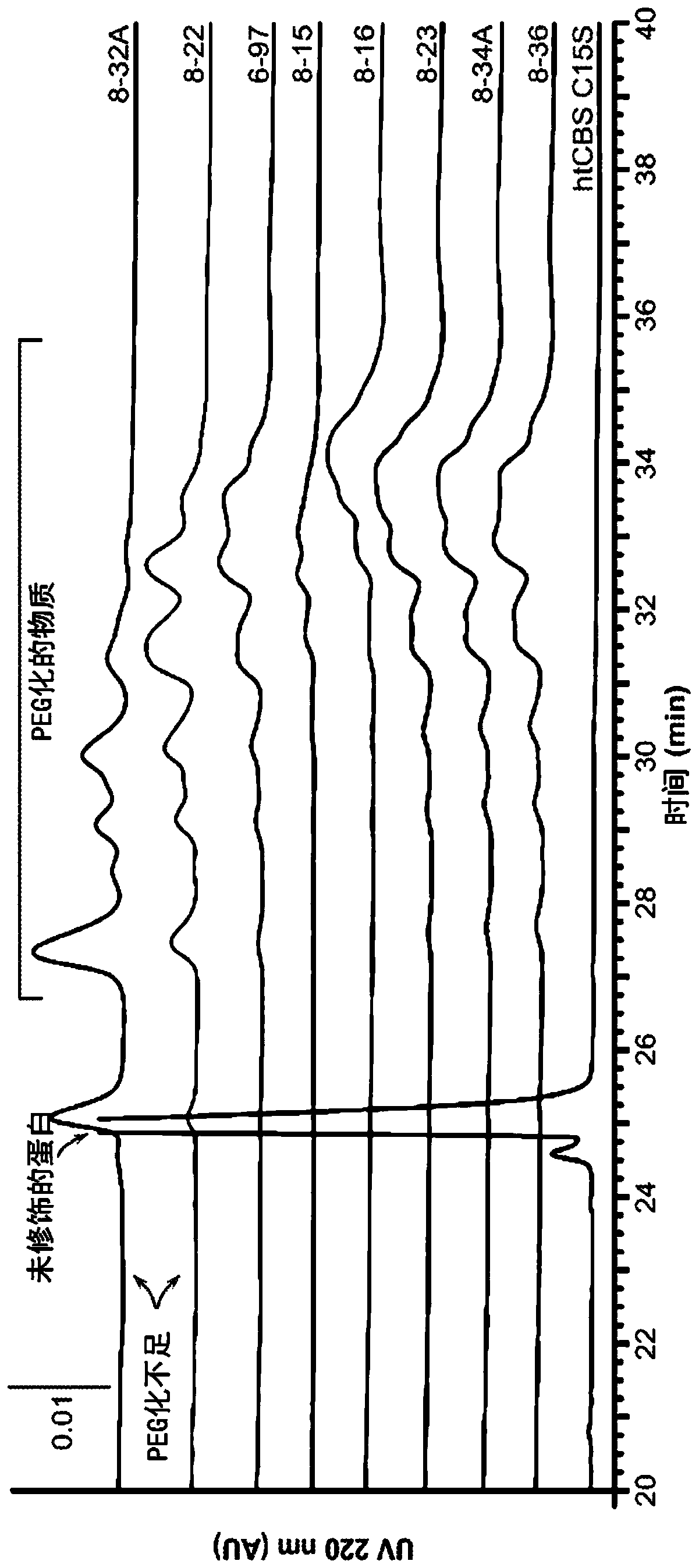
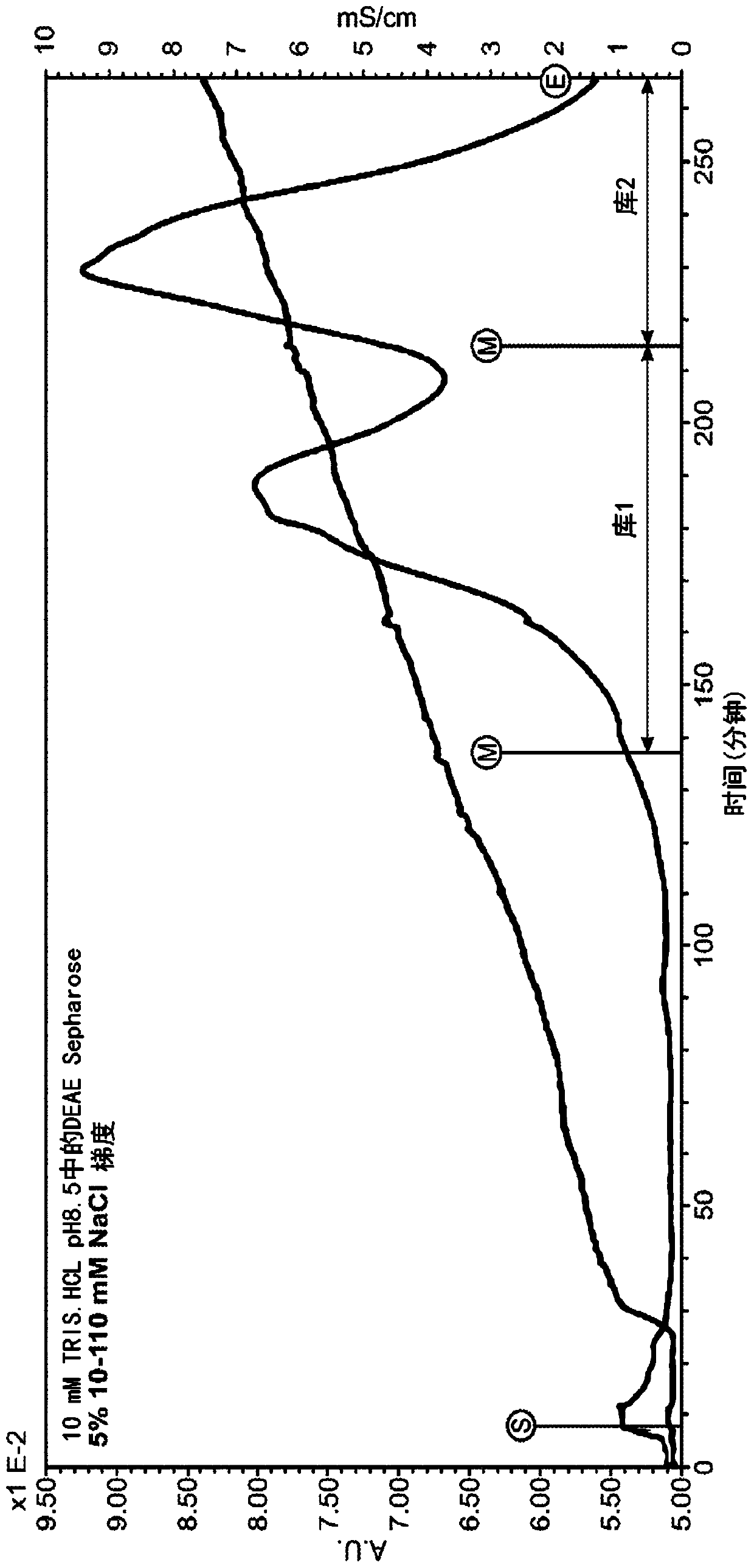
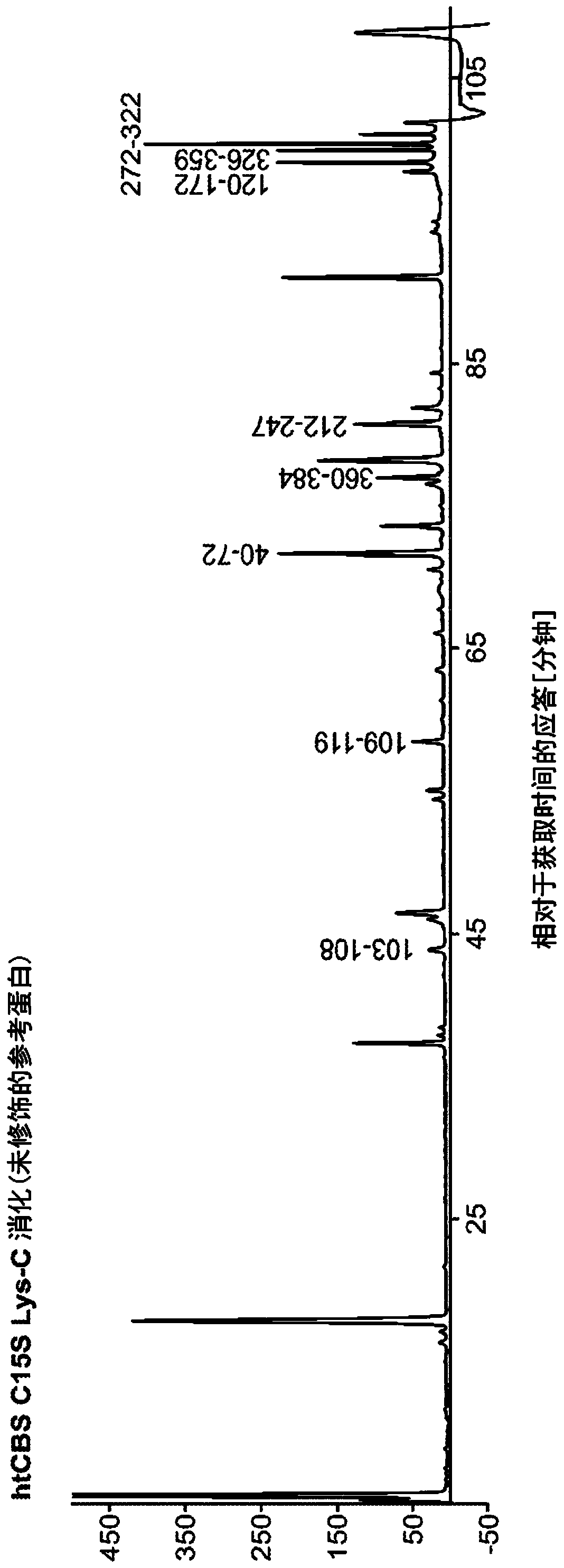
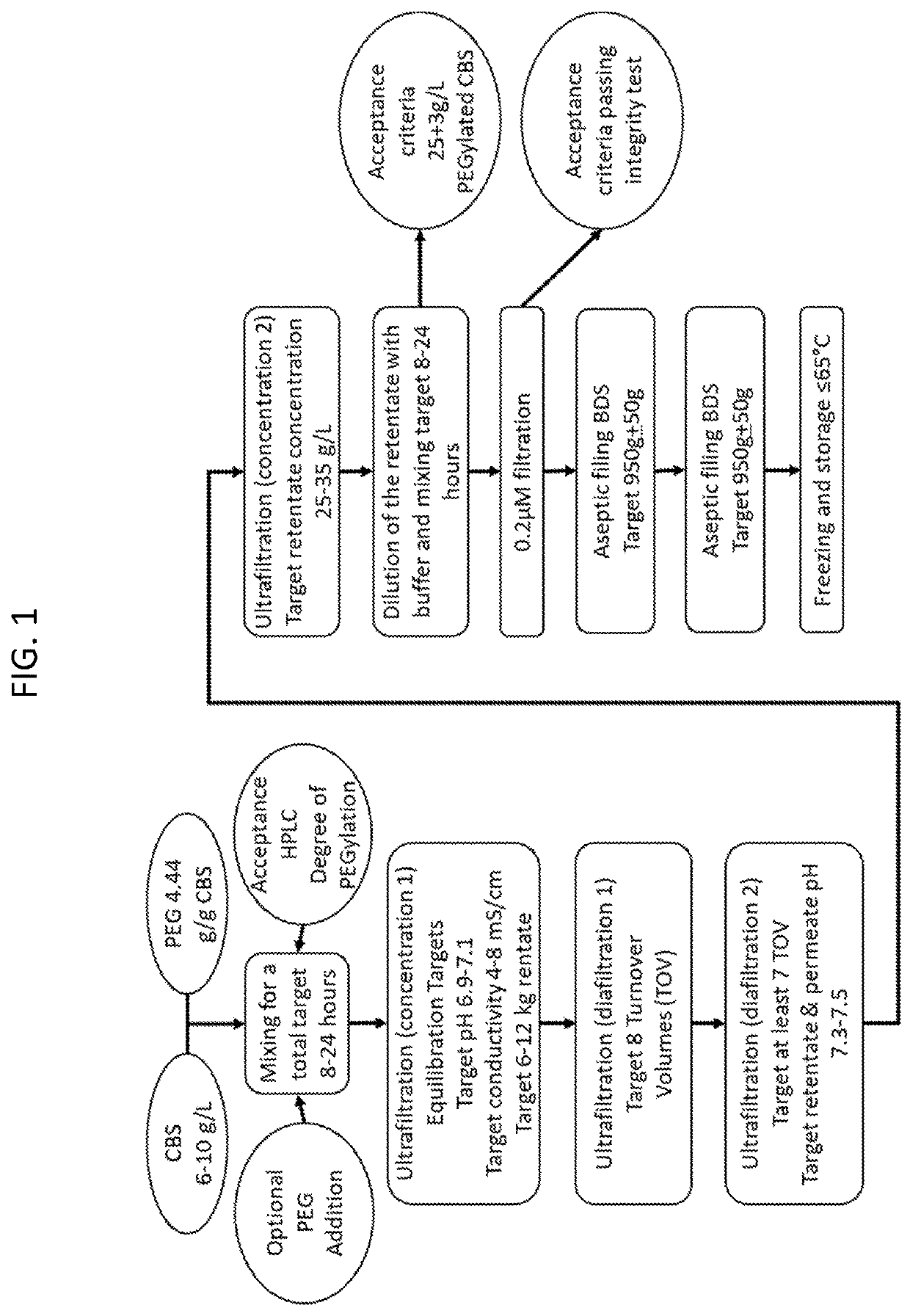
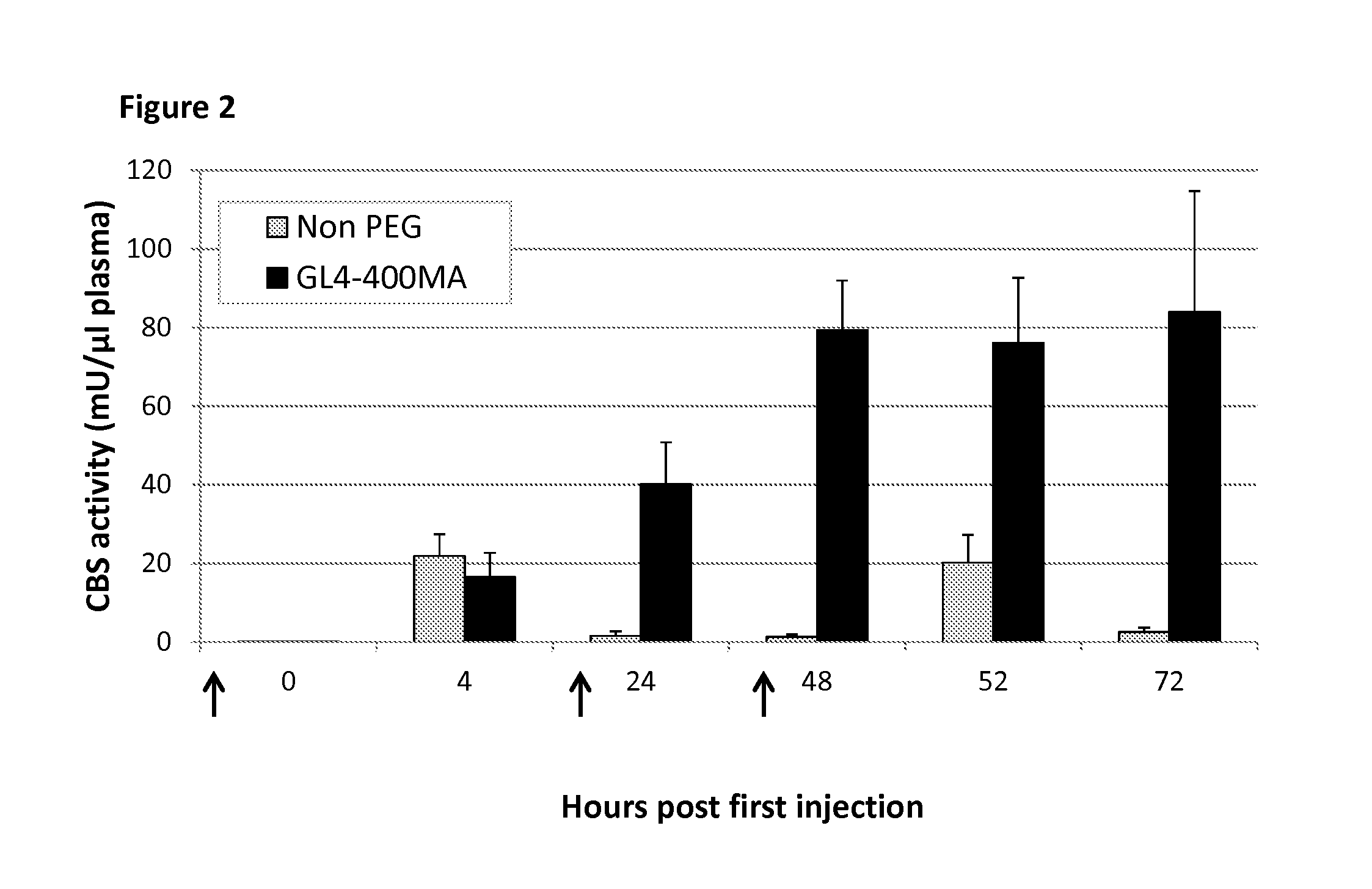
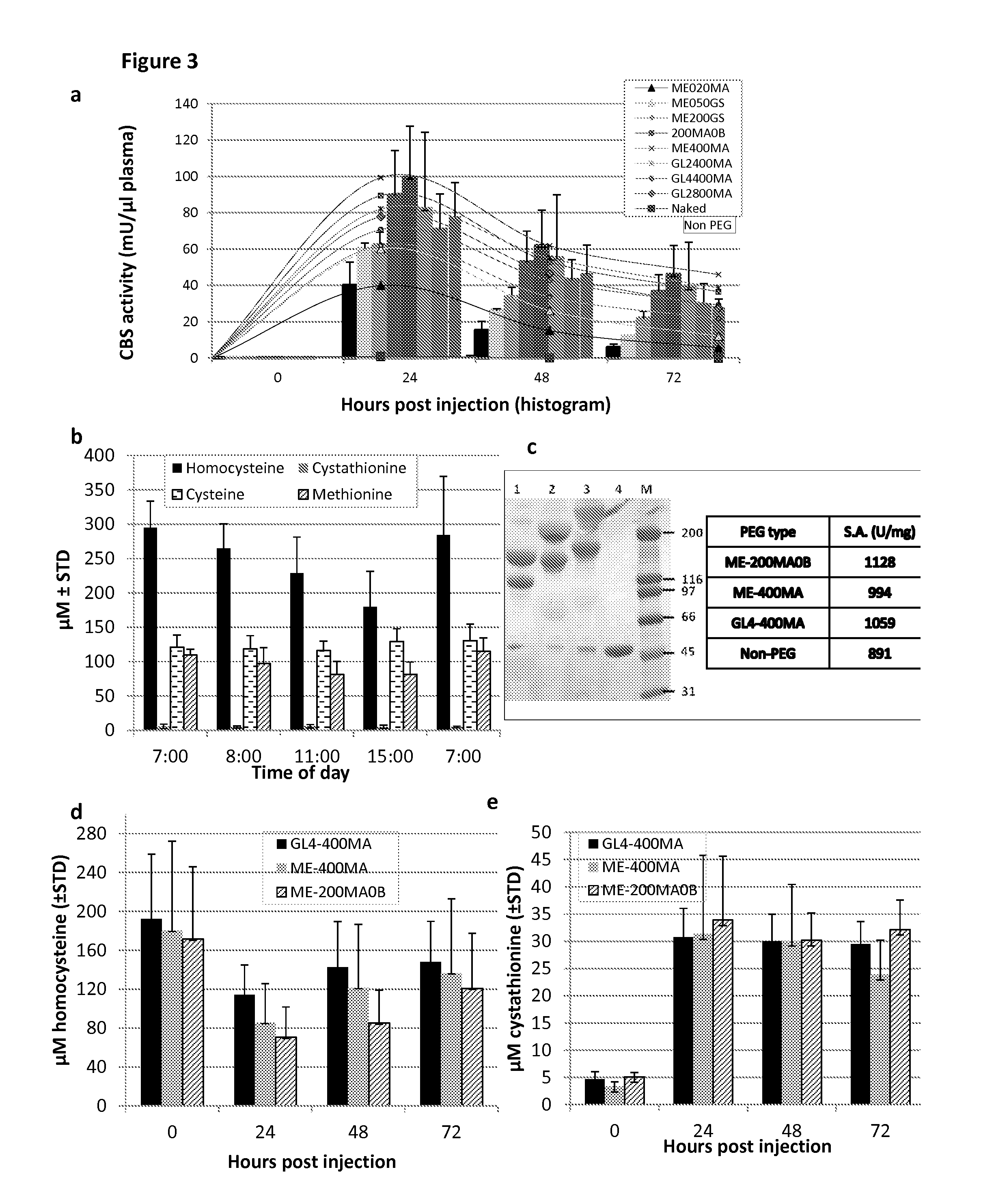
The present invention provides a method of PEGylating a human truncated cystathionine β-synthase protein containing a mutation of a cysteine to a serine at amino acid position 15 (htCBS C15S). The htCBS C15S was PEGylated with one of 5 kDa, 10 kDa, or 20 kDa NHS ester PEG molecules. In-process monitoring of the PEGylation process was used in the method to reduce levels of unPEGylated htCBS C15S and htCBS C15S with insufficient PEGylation. Administration of the PEGylated htCBS C15S had efficacy throughout the course of treatment for homocystinuria.
Owner:UNIV OF COLORADO THE REGENTS OF +1
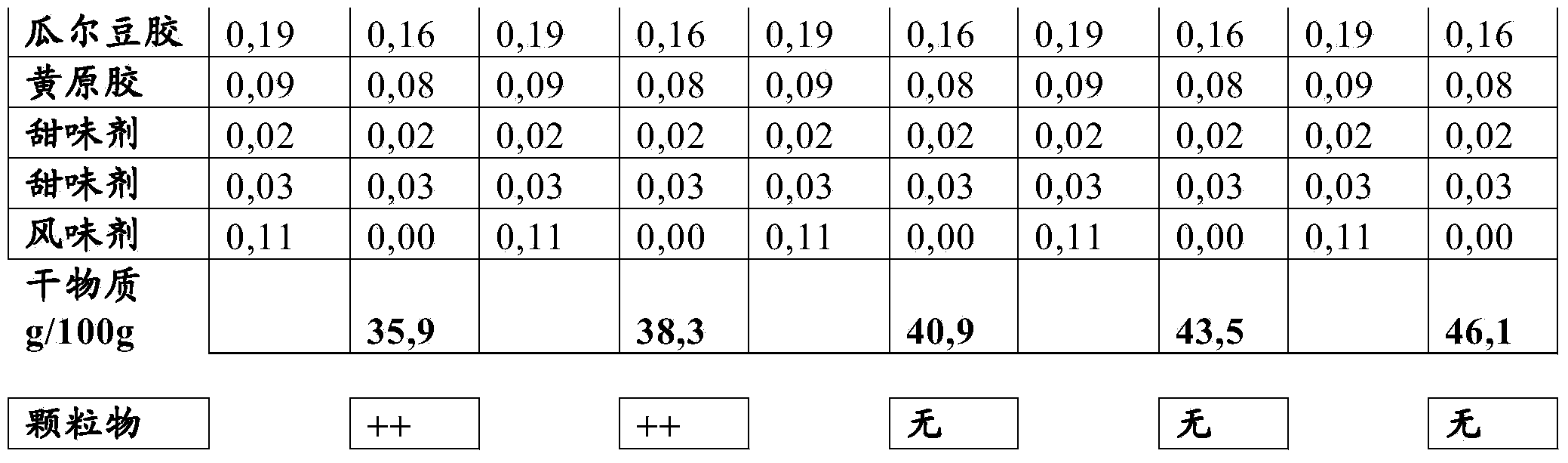
Liquid nutritional composition comprising free amino acids
InactiveCN104284602ASatisfied with the shelf lifeOrganic active ingredientsSugar food ingredientsDiseaseMethylmalonic acidaemia
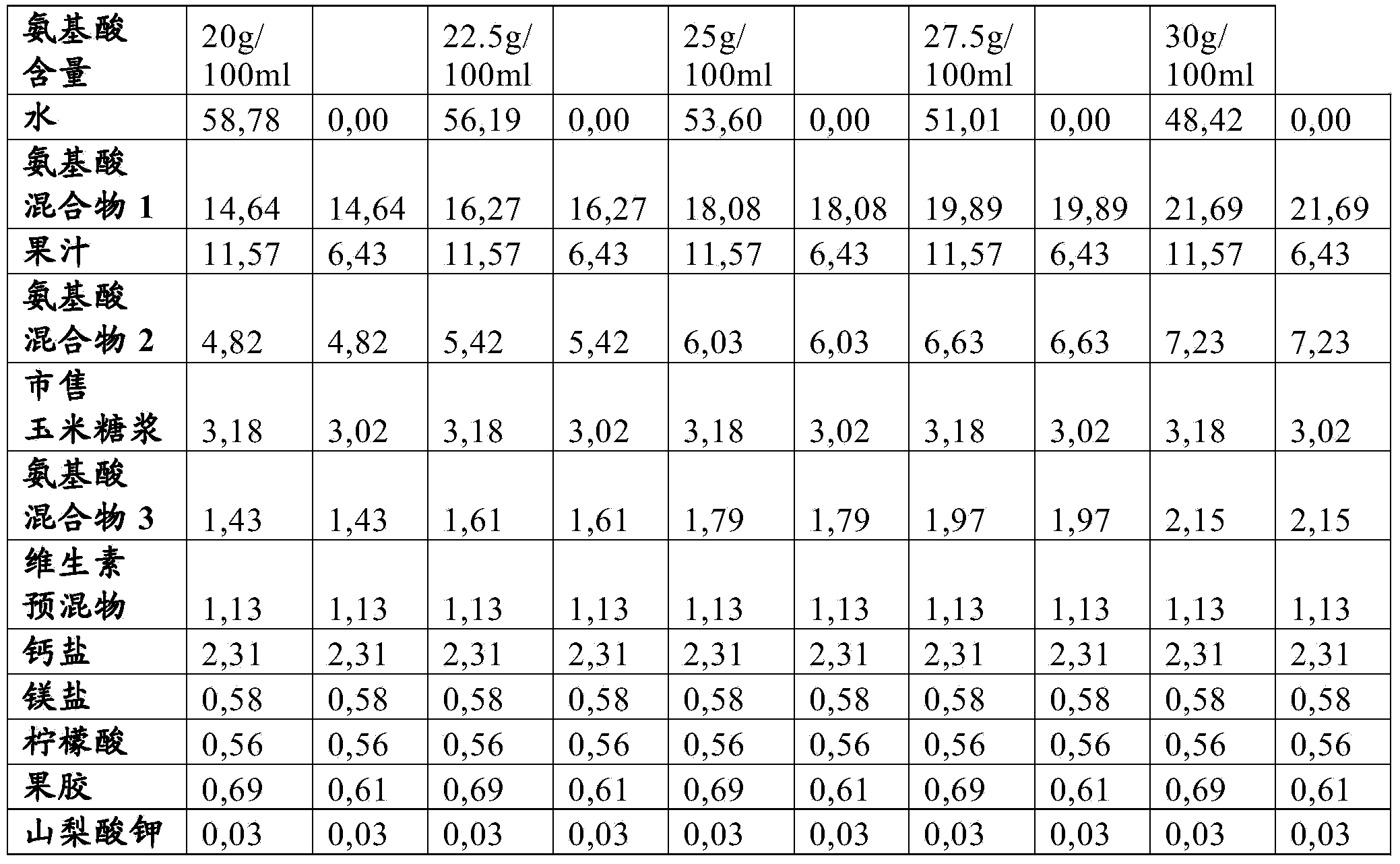
The invention relates to a liquid or gelled pasteurized or sterilized composition comprising free amino acids, calcium, a digestible carbohydrate, and preferably an indigestible carbohydrate, wherein the free amino acids are present in a concentration between 5 and 30g / 100ml, and the dry weight of the total composition is at least 40g / 100ml, and preferably lower than 60 g / 100ml. The composition may in particular be used in the treatment of a human suffering from a disorder selected from the group consisting of phenylketonuria, homocystinuria, maple syrup urine disease, tyrosinaemia, propionic acidaemia, methylmalonic acidaemia, isovaleric acidaemia, urea cycle disorders and glutaric aciduria.
Owner:NV NUTRICIA
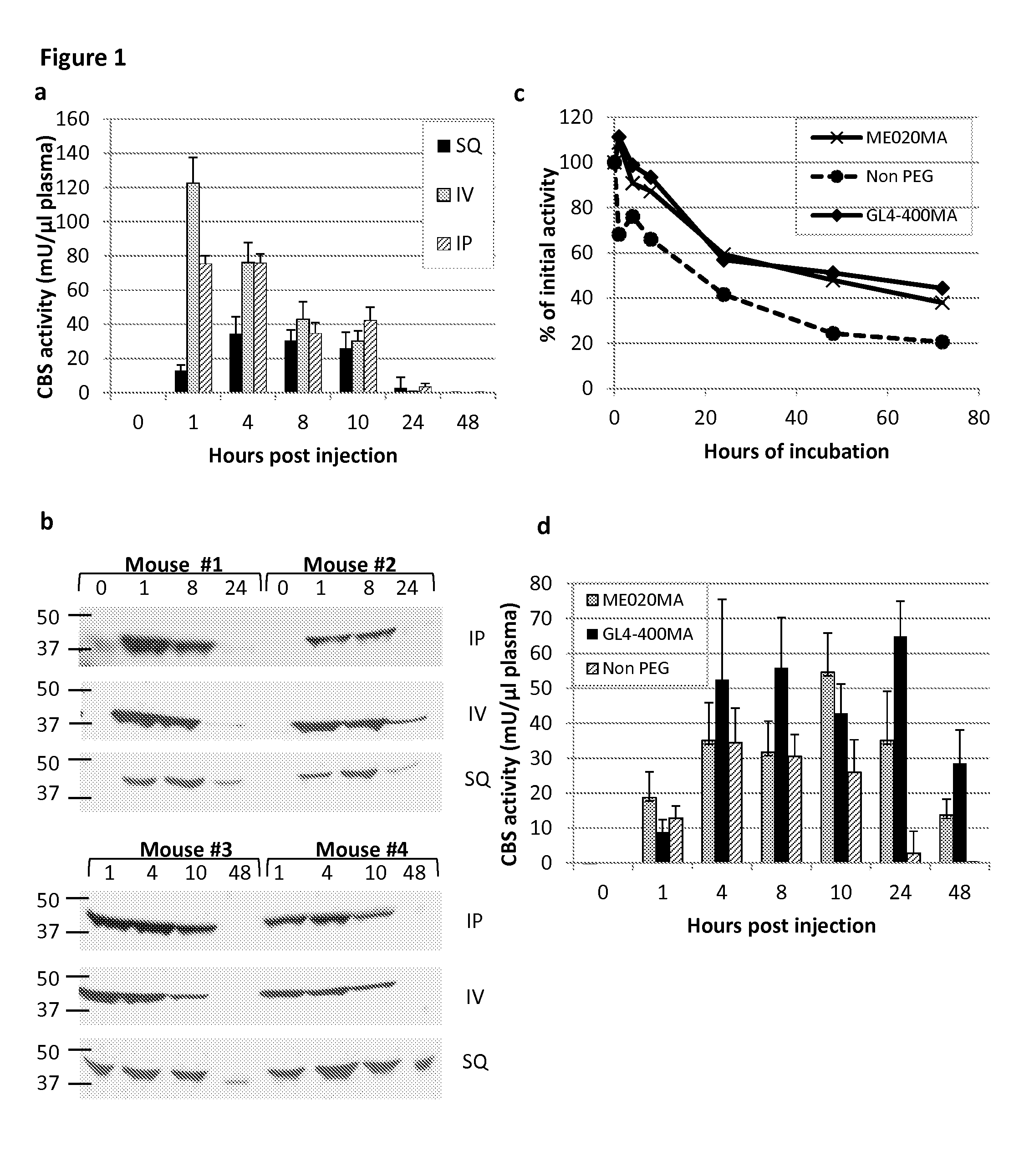
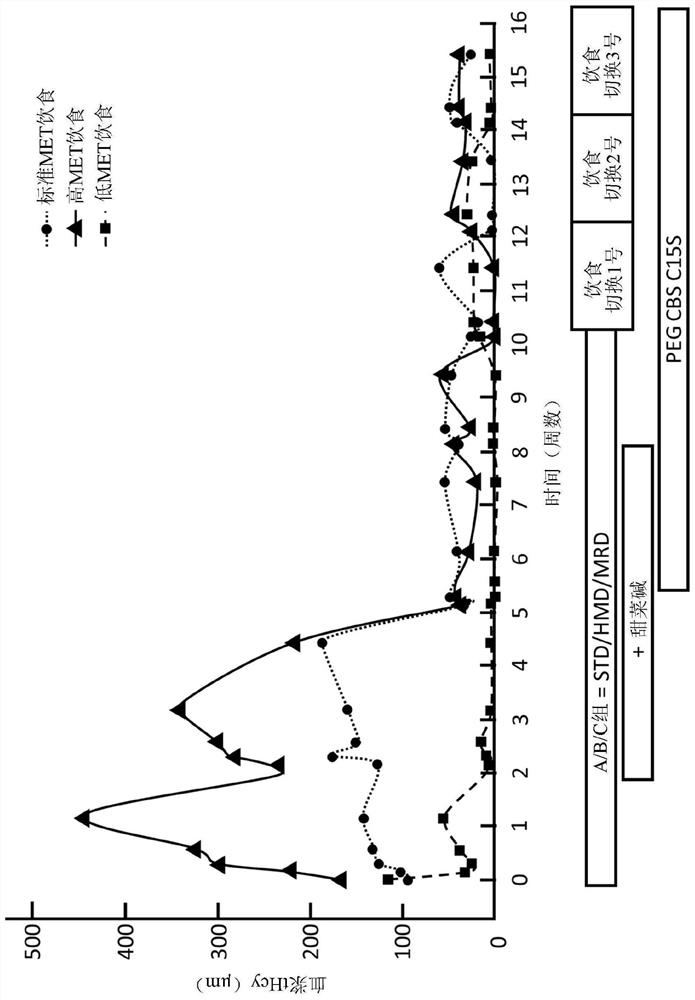
PEGylated cystathionine beta synthase for enzyme therapy for treatment of homocystinuria
Owner:特拉维尔治疗瑞士有限公司 +1
Compositions and methods for treatment of homocystinuria
Provided are compositions and methods for enzyme replacement therapy using modified human cystathionine beta synthase (CBS) in the treatment of homocystinuria and related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
Gene therapy for combined methylmalonic acidemia/aciduria and hyperhomocysteinemia/homocystinuria, cobalamin c type, and deficiency of mmachc
ActiveUS20180353623A1Genetic material ingredientsPharmaceutical non-active ingredientsHyperhomocystinemiaMMACHC
The present invention provides a synthetic MMACHC polynucleotide comprising a polynucleotide encoding MMACHC that is codon-optimized for expression in a human. Also provided is a polypeptide encoded by a synthetic MMACHC polynucleotide, an expression vector comprising a MMACHC gene sequence under the control of a chicken beta actin (CBA) promoter, and an expression vector comprising a synthetic MMACHC polynucleotide. Methods of treating cobalamin C deficiency and for detecting or tracking exogenous MMACHC are also provided.
Owner:UNITED STATES OF AMERICA
Uses of and methods of treatment with cystathionine
InactiveUS20180050004A1Reduce developmentGood curative effectOrganic active ingredientsUrinary disorderDiseaseMedicine
The present invention relates to uses of and methods of treatment with cystathionine. In one embodiment, cystathionine reduces the development of toxin-induced liver and / or kidney disease induced by homocystinuria and / or acute nephropathy. In one embodiment, a cystathionine synthesis inhibitor is administered to increase tumor cell apoptosis and / or increase the efficacy of chemotherapeutic treatment. More particularly, cystathionine can protect cells against toxin-induced cellular apoptosis and / or cystathionine synthesis inhibitors can increase neuroblastoma cell kill rates during chemotherapy.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Optimization of enzyme replacement therapy for treatment of homocystinuria
The present invention provides a method of PEGylating a human truncated cystathionine -synthase protein containing a mutation of a cysteine to a serine at amino acid position 15 (htCBS C15S). The htCBS C15S was PEGylated with one of 5 kDa, 10 kDa, or 20 kDa NHS ester PEG molecules. In-process monitoring of the PEGylation process was used in the method to reduce levels of unPEGylated htCBS C15S andhtCBS C15S with insufficient PEGylation. Administration of the PEGylated htCBS C15S had efficacy throughout the course of treatment for homocystinuria.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Pegylated cystathionine beta synthase for enzyme therapy for treatment of homocystinuria
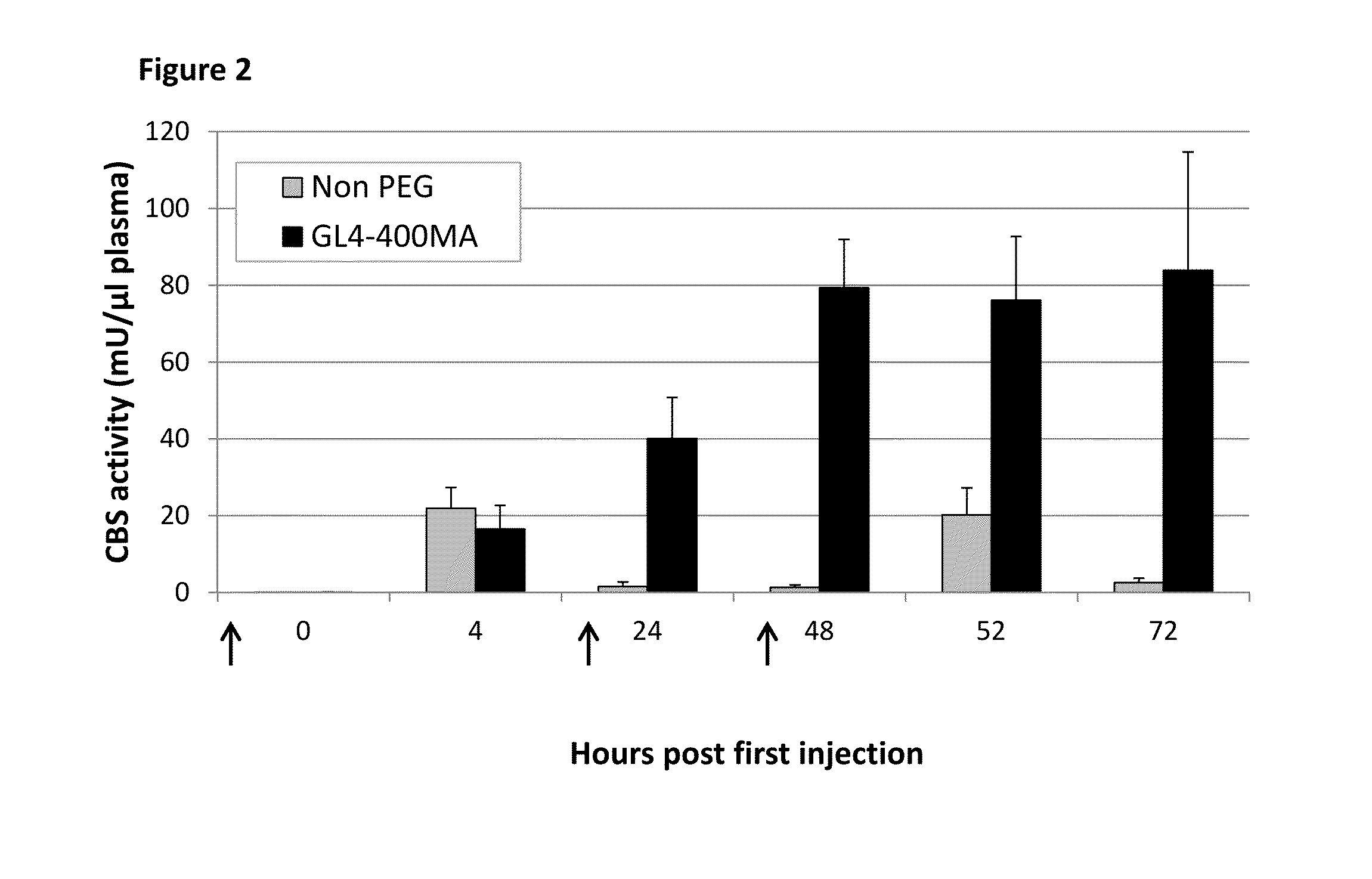
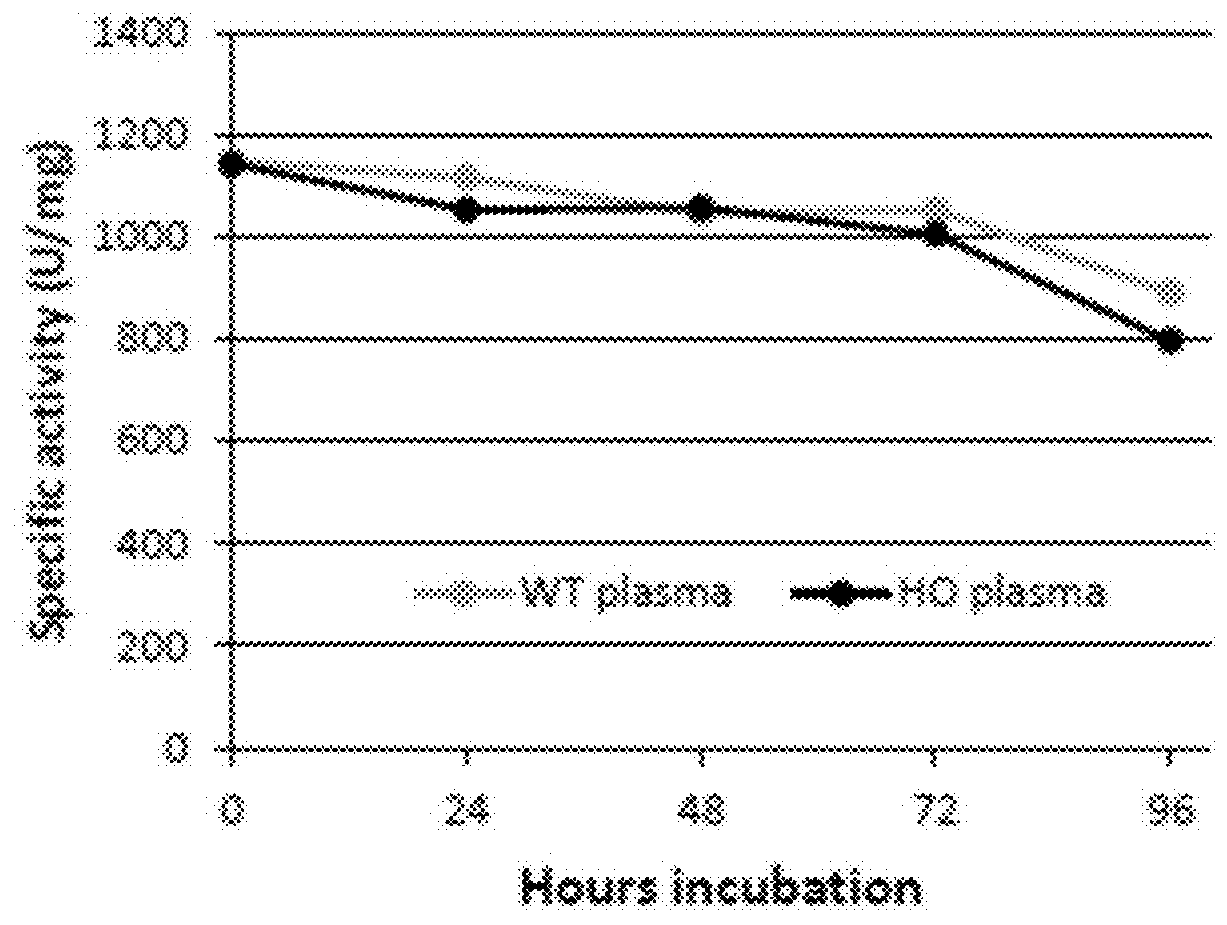
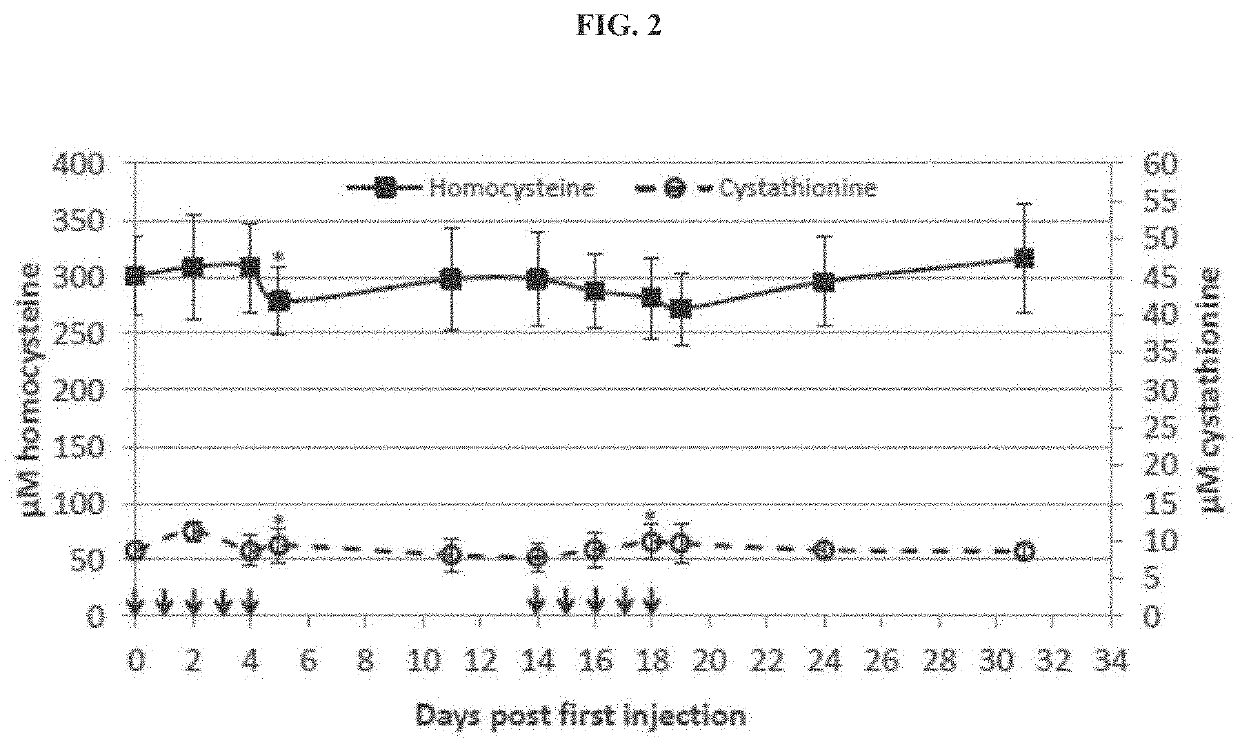
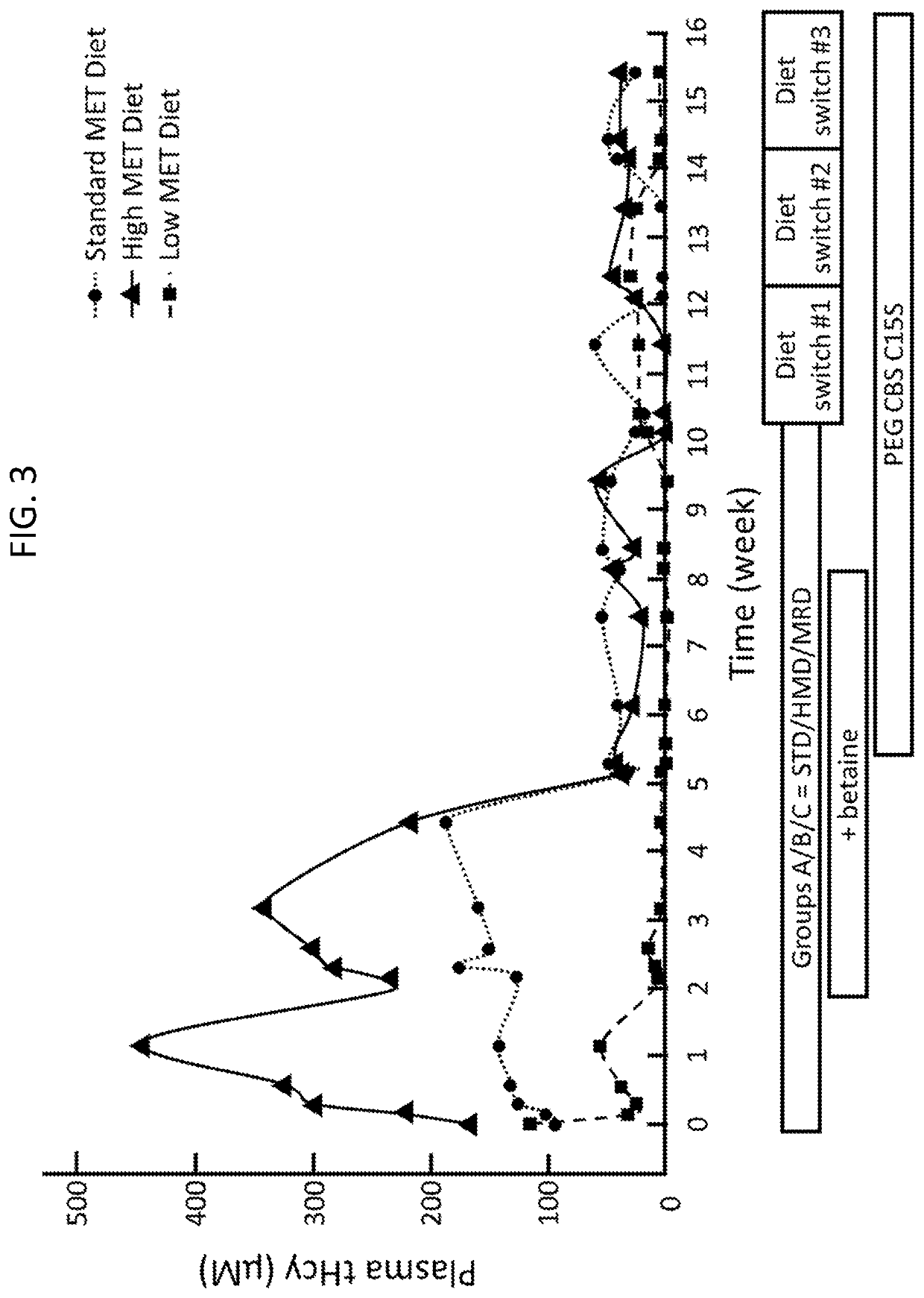
The present disclosure provides formulations for a drug product comprising a PEGylated CBS protein having the amino acid sequence of SEQ ID NO: 1. Dosages and dosing regimens are provided for treatment of homocystinuria in a subject in need thereof. Additionally, the dosages and dosing regimens are also provided to reduce the level of homocysteine (Hcy) or increase the levels of cysteine (Cys) and / or cystathionine (Cth) in a subject in need thereof.
Owner:TRAVERE THERAPEUTICS SWITZERLAND GMBH +1
Cystathionine beta-synthase enzyme therapy for treatment of elevated homocysteine levels
PendingUS20220290116A1Reduce vulnerabilityNervous disorderPeptide/protein ingredientsPhysiologyBehavioral interventions
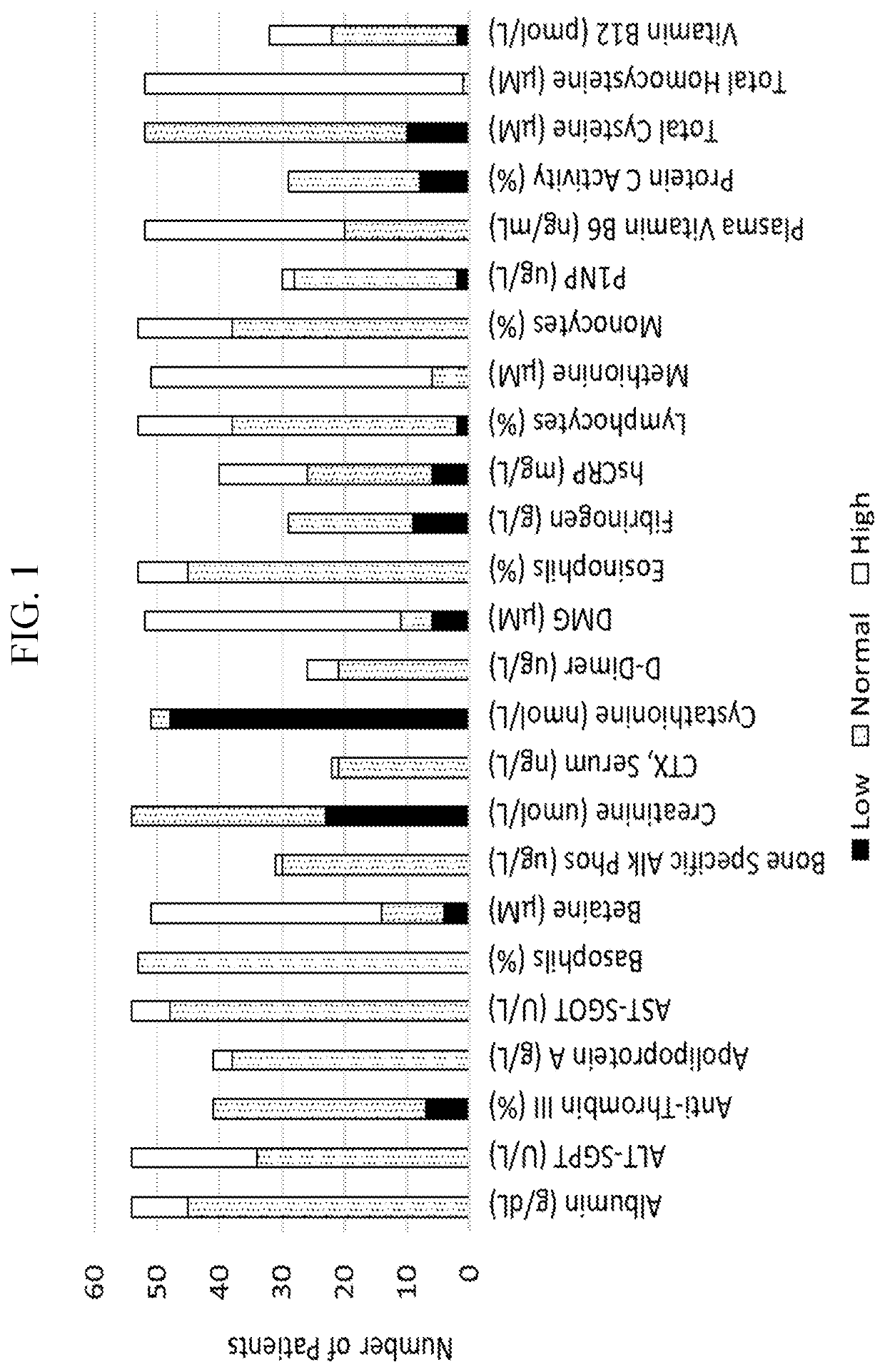
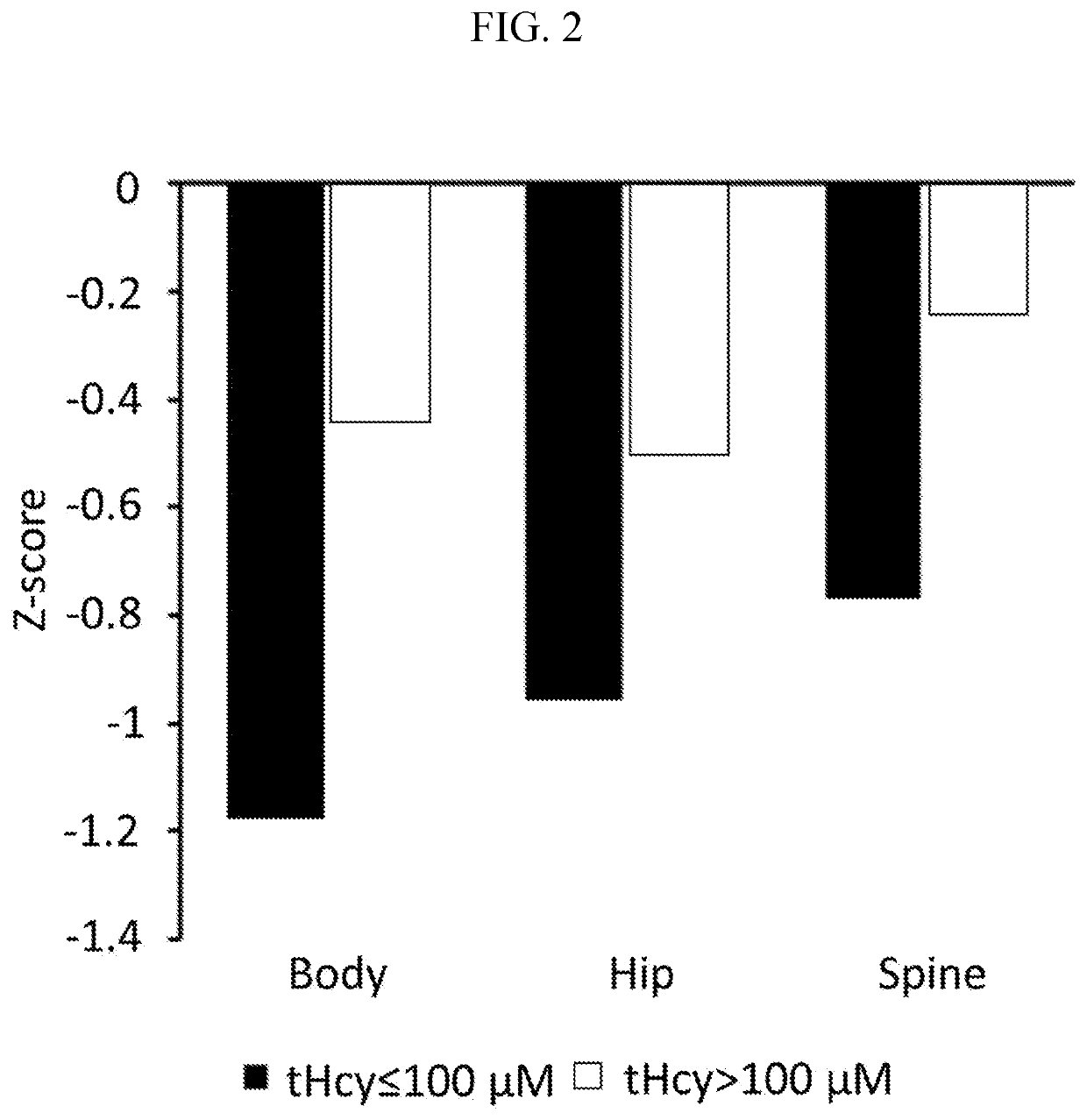
The present disclosure provides methods for treating homocystinuria or elevated homocysteine levels in subjects, including methods of improving cognitive function and ameliorating skeletal fragility, and methods of stratifying patient populations to determine disease progression or severity and / or to determine treatment regimens. In some embodiments, the methods of improving cognitive function in a subject having elevated total plasma homocysteine (tHcy) levels further comprise providing a cognitive or behavioral intervention.
Owner:TRAVERE THERAPEUTICS SWITZERLAND GMBH
Compositions and methods for treating homocystinuria and other conditions
PendingUS20220096408A1Improve clinical outcomesGood curative effectMetabolism disorderInorganic active ingredientsFormateEfficacy
Embodiments of the instant disclosure relate to novel compositions and methods for treating a subject having homocystinuria. In some embodiments, compositions and methods disclosed herein concern improving efficacy of betaine to reduce dietary compliance requirements and improve outcomes. In accordance with these embodiments, a subject having or suspected of developing homocystinuria can be treated with formate, a salt thereof, a formate derivative or formate precursor or prodrug agent alone or in combination with trimethylglycine or other HCU treatments. In other embodiments, a subject having or suspected of developing homocystinuria can be treated with zinc and / or trimethylglycine and / or formate derivative to treat homocystinuria in the subject. In other embodiments, a subject having or suspected of developing Nonketotic hyperglycinemia (NKH) can be treated with formate, a salt thereof, a formate derivative, or formate precursor or prodrug agent to treat NKH in the subject.
Owner:UNIV OF COLORADO THE REGENTS OF
Uses of and methods of treatment with cystathionine
InactiveUS20140163104A1Reduce developmentGood curative effectOrganic active ingredientsBiocideDiseaseTherapeutic treatment
The present invention relates to uses of and methods of treatment with cystathionine. In one embodiment, cystathionine reduces the development of toxin-induced liver and / or kidney disease induced by homocystinuria and / or acute nephropathy. In one embodiment, a cystathionine synthesis inhibitor is administered to increase tumor cell apoptosis and / or increase the efficacy of chemo therapeutic treatment. More particularly, cystathionine can protect cells against toxin-induced cellular apoptosis and / or cystathionine synthesis inhibitors can increase neuroblastoma cell kill rates during chemotherapy.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Chemically modified cystathionine beta-synthase enzyme for treatment of homocystinuria
ActiveUS20150344864A1Reducing serum HcyHigh activityOrganic active ingredientsSenses disorderMedicineCystinuria
The invention provides reagents and methods for enzyme replacement therapy using chemically modified species of human cystathionine β-synthase (CBS) to treat homocystinuria and other related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
Human-enzyme mediated depletion of homocysteine for treating patients with hyperhomocysteinemia and homocystinuria
ActiveUS11033612B2Lower Level RequirementsEfficient degradationOrganic active ingredientsPowder deliveryHyperhomocystinemiaAmino acid substitution
Methods and compositions relating to the engineering of an improved protein with homocyst(e)inase enzyme activity are described. For example, there are disclosed modified cystathionine-γ-lyase (CGL) enzymes comprising one or more amino acid substitutions and capable of degrading homocyst(e)ine. Furthermore, provided are compositions and methods for the treatment of homocystinuria or hyperhomocysteinemia with homocyst(e)ine depletion using the disclosed enzymes or nucleic acids.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com