Method of preparing group III-V compound semiconductor crystal
a technology of compound semiconductor crystals and semiconductor crystals, which is applied in the direction of polycrystalline material growth, under a protective fluid, gel state, etc., can solve the problems of difficult control of carbon concentration, inability to adjust carbon concentration, and impurity contamination, so as to achieve greater effect, increase purity, and prolong heat treatment time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
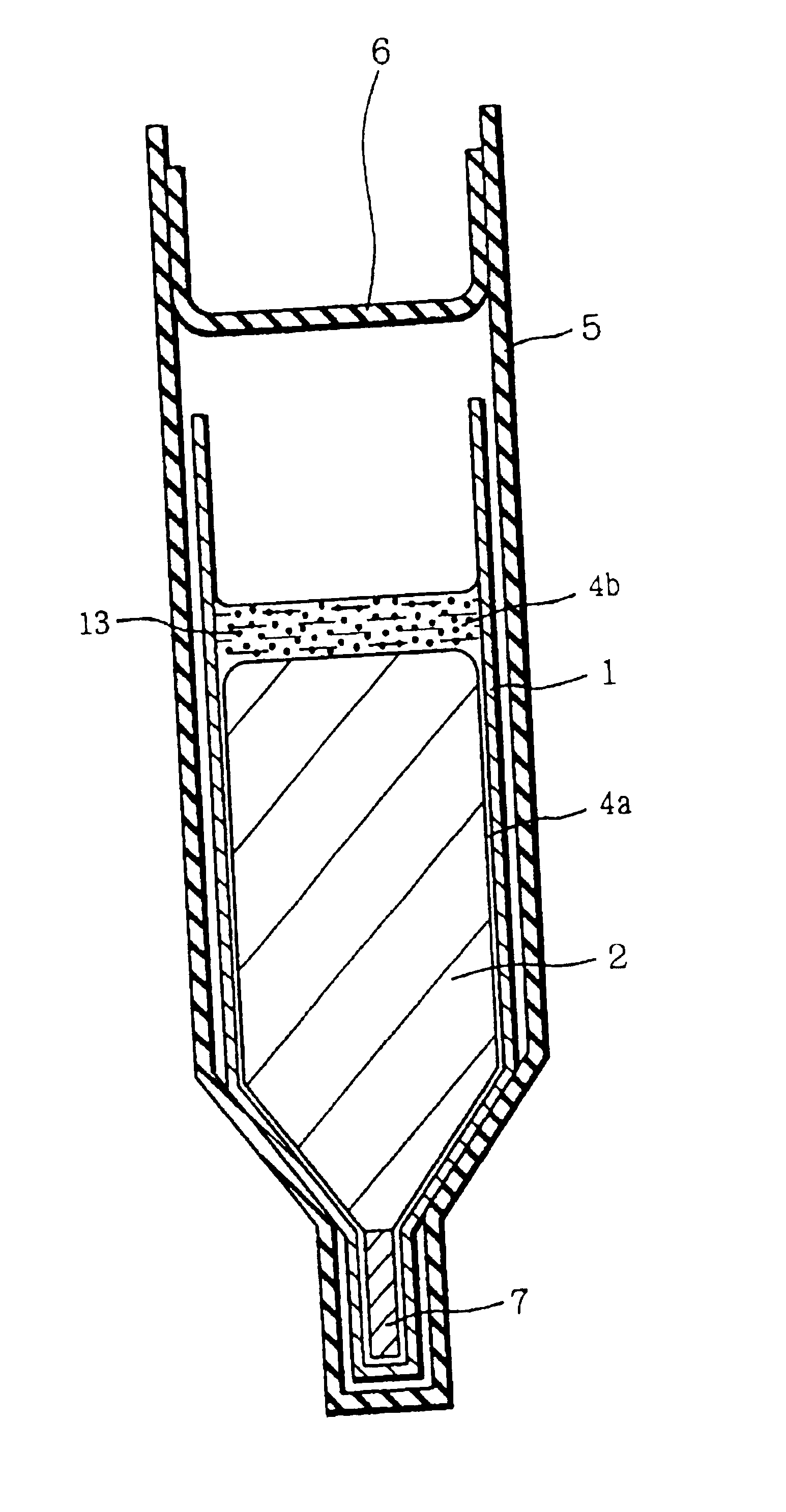
[0045]FIG. 1 is a diagram for describing an example of preparing a group III-V compound semiconductor crystal according to the present invention. Referring to FIG. 1, GaAs polycrystalline raw material 2, carbon powder 13 which has been subjected to heat treatment under reduced pressure in advance, boron oxide (B2O3) 4 forming a boron oxide layer 4b and a boron oxide film 4a as described below, and a seed crystal 7 were placed in a pBN crucible 1. The seed crystal 7 was placed at the bottom portion of the crucible 1. In crucible 1, the materials were so arranged that carbon powder 13 and boron oxide 4 were brought into contact with each other, and also boron oxide 4 and raw material 2 were brought into contact with each other when the raw material was melted.
[0046]Crucible 1 was inserted in a quartz ampoule 5 together with solid arsenic. Ampoule 5 was sealed under reduced pressure with a quartz cap 6.
[0047]Respective conditions of Example 1 are shown in the following Table 1.
[0048]
TA...
example 2
[0054]FIG. 3 is a diagram for describing another example of a method of preparing a group III-V compound semiconductor crystal of the present invention. Referring to FIG. 3, GaAs polycrystalline raw material 2, carbon fiber 23 subjected to heat treatment under reduced pressure in advance, boron oxide 4 forming a boron oxide layer 4b and a boron oxide film 4a as described below, and a seed crystal 7 were placed in a pBN crucible 1. Seed crystal 7 was placed at the bottom portion of the crucible 1. In crucible 1, the materials were arranged so that carbon fiber 23 and boron oxide 4 were brought into contact with each other and also boron oxide 4 and raw material 2 were brought into contact with each other when the raw material was melted. Crucible 1 was inserted into a quartz ampoule 5 together with solid arsenic. Quartz ampoule 5 was sealed under reduced pressure with a quartz cap 6. Respective conditions of Example 2 are shown in the following Table 3.
[0055]
TABLE 3GaAs10 kg usedpoly...
example 3
[0059]A carbon-doped GaAs single crystal was grown using 20 mg of carbon fiber similar to that of Example 2. The other conditions of the experiment were identical to those of Example 2, and their description will not be repeated. The characteristics of the obtained single crystal are shown in the following Table 5.
[0060]
TABLE 5Crystal diameter105 mmLength of φ105 mm portion200 mmCarbon concentrationShoulder2.3 × 1015 cm−3Tail2.2 × 1015 cm−3ResistivityShoulder8.8 × 107 ΩcmTail8.4 × 107 ΩcmDislocation densityShoulder1000 cm−2Tail1800 cm−2
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| grain size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com