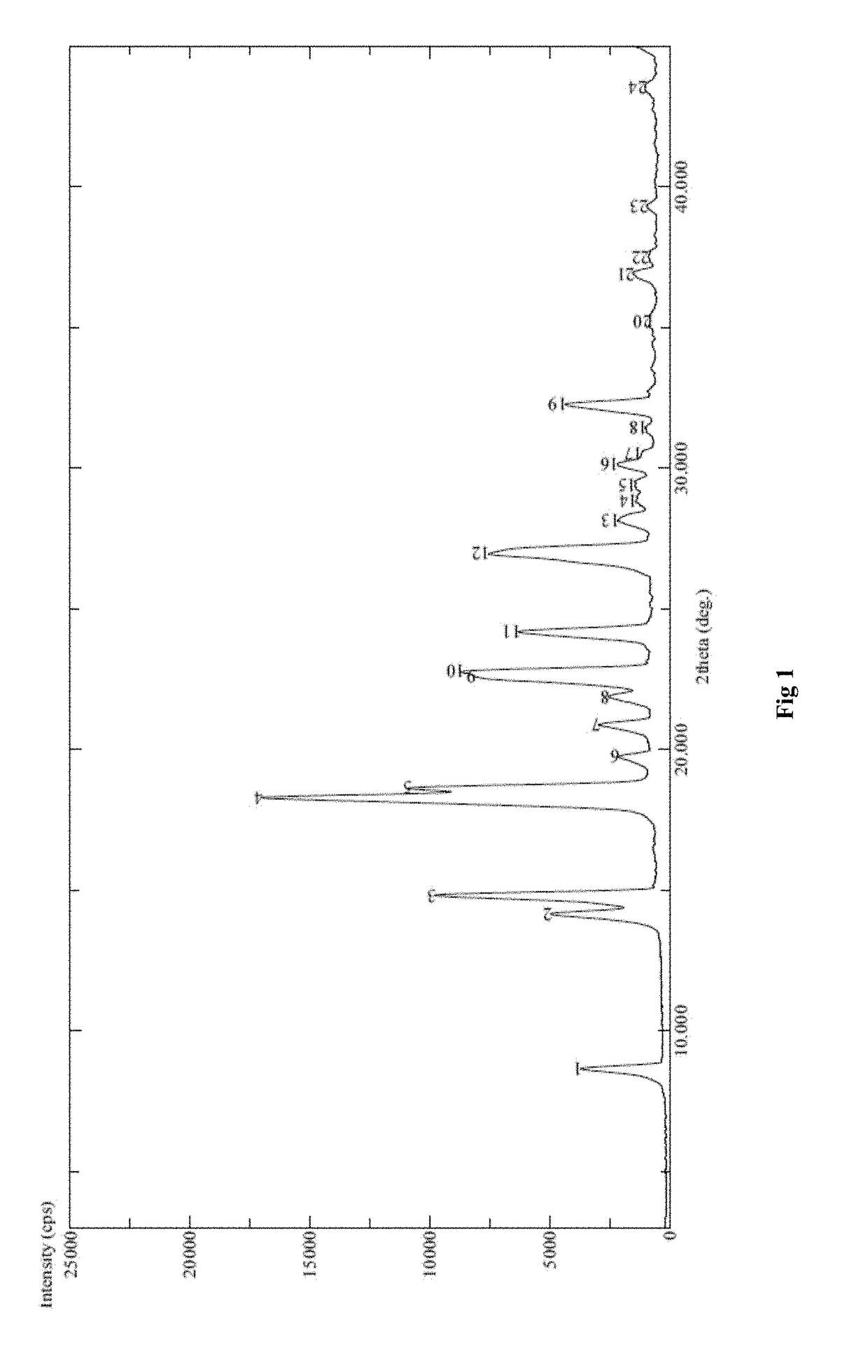
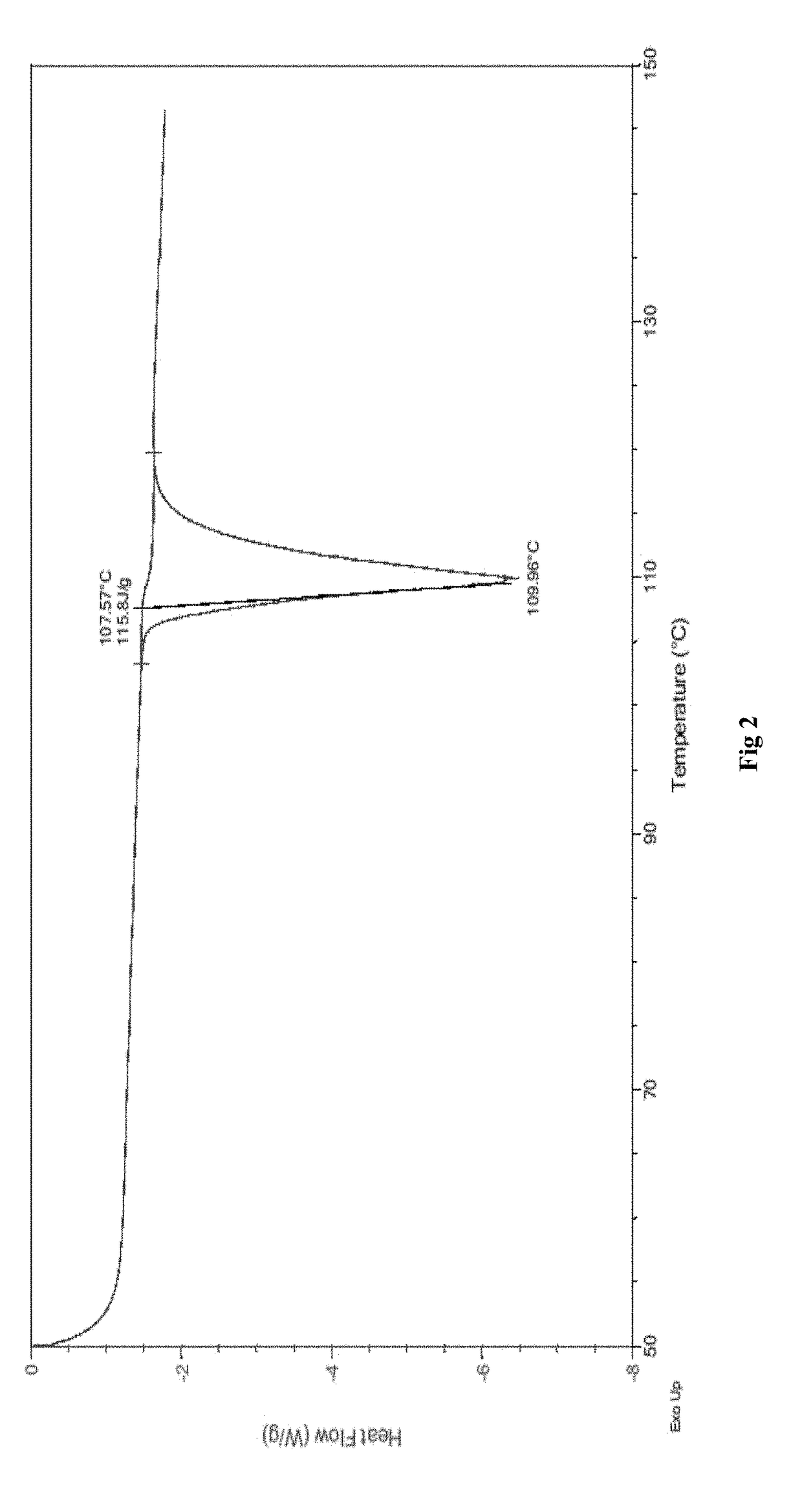
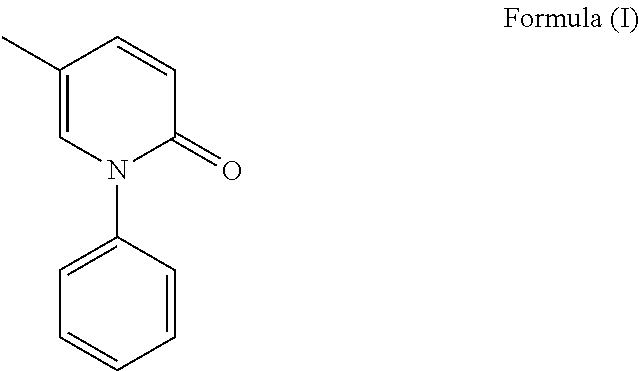
Process for the preparation and particle size reduction of pirfenidone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 5-methyl-1H-pyridin-2-one
[0107]DM water (800 ml) was added to concentrated sulfuric acid (227 g) at 0-5° C. and stirred for 15 mins. To this, 2-amino-5-methyl pyridine (100 g) followed by aqueous sodium nitrite (83 g of sodium nitrite dissolved in 200 ml of water) was added at below 10° C. The reaction mass was stirred for an hour at 0-5° C. After the reaction completion, reaction mass temperature was raised to 25-35° C. and stirred for 4 hrs. Aqueous sulphamic acid (26.9 g of sulphamic acid dissolved in 100 ml of water) was added to the reaction mass and stirred for 60 mins at 25-35° C. The reaction mass was cooled to 10-15° C. and pH was adjusted to 7 with aqueous sodium hydroxide solution. The reaction mass was heated to 55-65° C. and extracted with ethyl acetate (6×500 ml). The solvent from the extract was distilled off completely under mild vacuum at below 60° C. Ethyl acetate (200 ml) was added to the obtained residue and the reaction mixture was cooled to 25-35...
example 2
Preparation of Pirfenidone
[0109]A mixture of 5-methyl-1H-pyridin-2-one (100 g), bromo benzene (259 g, comprising greater than 0.2% by weight dibromobenzene isomers) and dimethylformamide (200 ml) were added in to a round bottom flask and stirred up to complete dissolution. Potassium carbonate (254 g) and copper (I) chloride (18.2 g) was added to the above reaction mass and then heated to 130-140° C. The reaction mass was stirred at 130-140° C. for 10 hrs. After the reaction completion, the reaction mass was cooled to 25-35° C. Toluene (500 ml), aqueous sodium chloride (75 g of sodium chloride in 500 ml of water) was added to the reaction mass and stirred for 15-30 mins at 25-35° C. The reaction mass was filtered and the filtrate was allowed to settle. Organic and aqueous layers were separated and the aqueous layer was extracted with toluene. Organic layers combined and was washed with aqueous sodium chloride, treated with carbon and filtered through hyflo. The solvent from the filtr...
example 3
Purification of Pirfenidone (from ethyl acetate).
[0114]A suspension of crude Pirfenidone (50g), contaminated with the dimer impurity (˜0.14% by HPLC) in ethyl acetate (100 mL) was maintained at 65-75° C. till the material completely dissolved. The reaction mass was treated with activated carbon (5g) and filtered through a short bed of Hyflo. The flask was rinsed with hot ethyl acetate (50 mL) and the combined filtrate was partially concentrated, under reduced pressure (till ˜2.0 volumes remaining in the flask), while maintain temperature below 60° C. The mixture was gradually cooled to 30±5° C. and then stirred for another 30-60 min. The suspension was cooled to 0-5° C. and maintained for another 2-3 h, at the same temperature. The precipitated material was filtered and then dried at 60±5° C., for 6-8 h, to afford Pirfenidone as white colored powder. Yield: 45 g, HPLC purity 99.9%; dimer content 0.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com