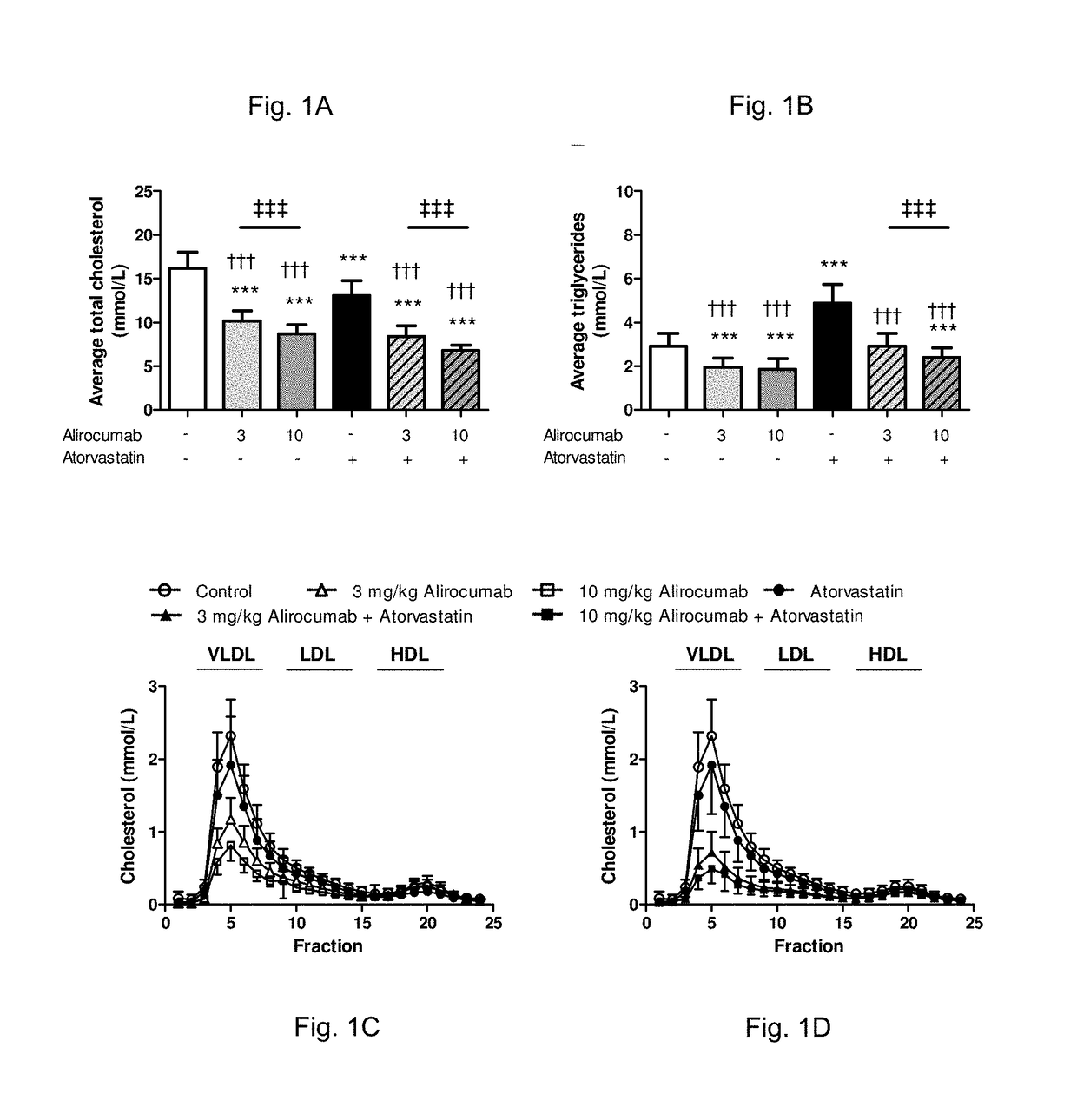
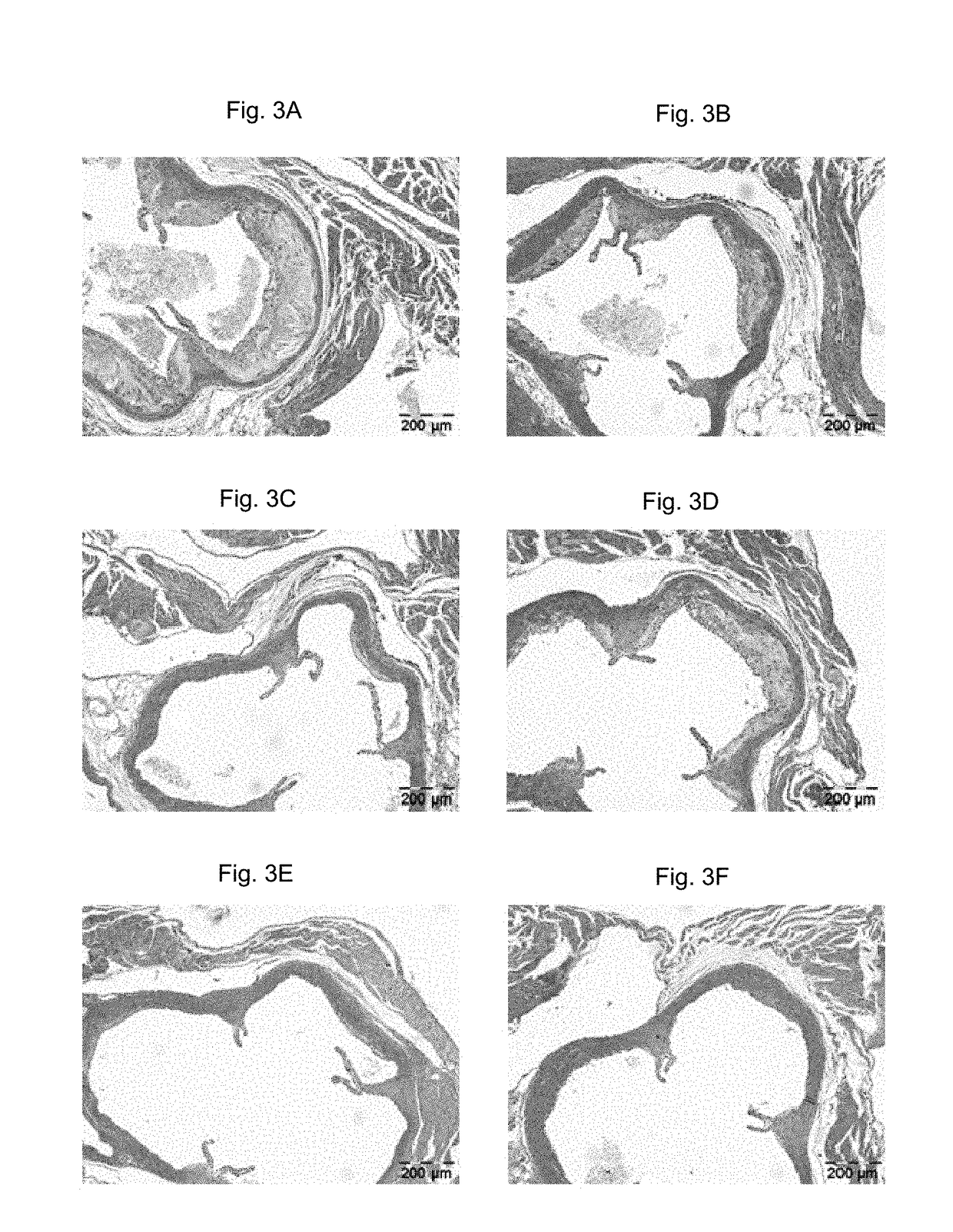
Methods for inhibiting atherosclerosis by administering an inhibitor of pcsk9
a technology of atherosclerosis and inhibitors, which is applied in the field of proproteins, can solve the problems that pcsk9 inhibitors have not been reported to reduce or inhibit the progression of atherosclerotic plaque formation, and achieve the effect of reducing atherosclerotic plaque formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Human Antibodies to Human PCSK9
[0133]Human anti-PCSK9 antibodies were generated as described in U.S. Pat. No. 8,062,640. The exemplary PCSK9 inhibitor used in the following Example is the human anti-PCSK9 antibody designated “mAb316P,” also known here as “alirocumab.” mAb316P has the following amino acid sequence characteristics: heavy chain variable region (HCVR) comprising SEQ ID NO:1; light chain variable domain (LCVR) comprising SEQ ID NO:6; heavy chain complementarity determining region 1 (HCDR1) comprising SEQ ID NO:2; HCDR2 comprising SEQ ID NO:3; HCDR3 comprising SEQ ID NO:4; light chain complementarity determining region 1 (LCDR1) comprising SEQ ID NO:7; LCDR2 comprising SEQ ID NO:8; and LCDR3 comprising SEQ ID NO:10.
example 2
Attainment of Low-Density Lipoprotein Cholesterol Goals in Patients at High Cardiovascular Risk: Results from a Managed Care Population Study
[0134]Background: US guidelines support LDL-C goals based on patient's cardiovascular (CV) risk profile, however, little is known regarding real-world patterns of LDL-C goal attainment within specific high CV risk conditions.[0135]Methods: Patients from the Optumlnsight IMPACT Database (a large US multi-payer claims database) with an LDL-C measurement during July 2011 to June 2012 and high CV risk conditions were identified. Most recent LDL-C measurement was defined as the index date and high CV risk conditions were identified hierarchically during the pre-index period as follows: recent acute coronary syndrome (ACS, within 6 months pre-index date), coronary events (myocardial infarction, hospitalization for unstable angina, coronary revascularization), stroke, and peripheral vascular disease (PVD).[0136]Results: In total, 110,739 patients met ...
example 3
Low-Density Lipoprotein Cholesterol Goal Attainment and Lipid-Lowering Therapy in a High Cardiovascular Risk Managed Care Population
[0138]Background: While US guidelines support statins as first line therapy to reduce LDL-C, little is known regarding real-world patterns of LDL-C goal attainment with statins and other lipid-lowering therapies (LLTs).[0139]Methods: Patients from the OptumInsight IMPACT Database (a large US multi-payer claims database) with an LDL-C measurement during July 2011 to June 2012 and high risk CV conditions (coronary events (myocardial infarction, hospitalization for unstable angina, coronary revascularization), stroke, and peripheral vascular disease) were identified. LLT prescription was assessed as of most recent LDL-C measurement (index date) and categorized as high-potency statin (atorvastatin 40 / 80 mg, rosuvastatin 20 / 40 mg, simvastatin 80 mg), standard-potency statin (other statins), non-statin LLT (ezetimibe, niacin, fibrates, bile acid sequestrants)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| pharmaceutical compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com