Metal fluoride coated lithium intercalation material and methods of making same and uses thereof
a lithium intercalation material and fluoride coating technology, applied in the field of electrochemistry, can solve the problems of reducing affecting the discharge/recharge cycle number, so as to reduce the charge/discharge capacity fade rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MgF2 Coated Spinel-Type Cathode Material
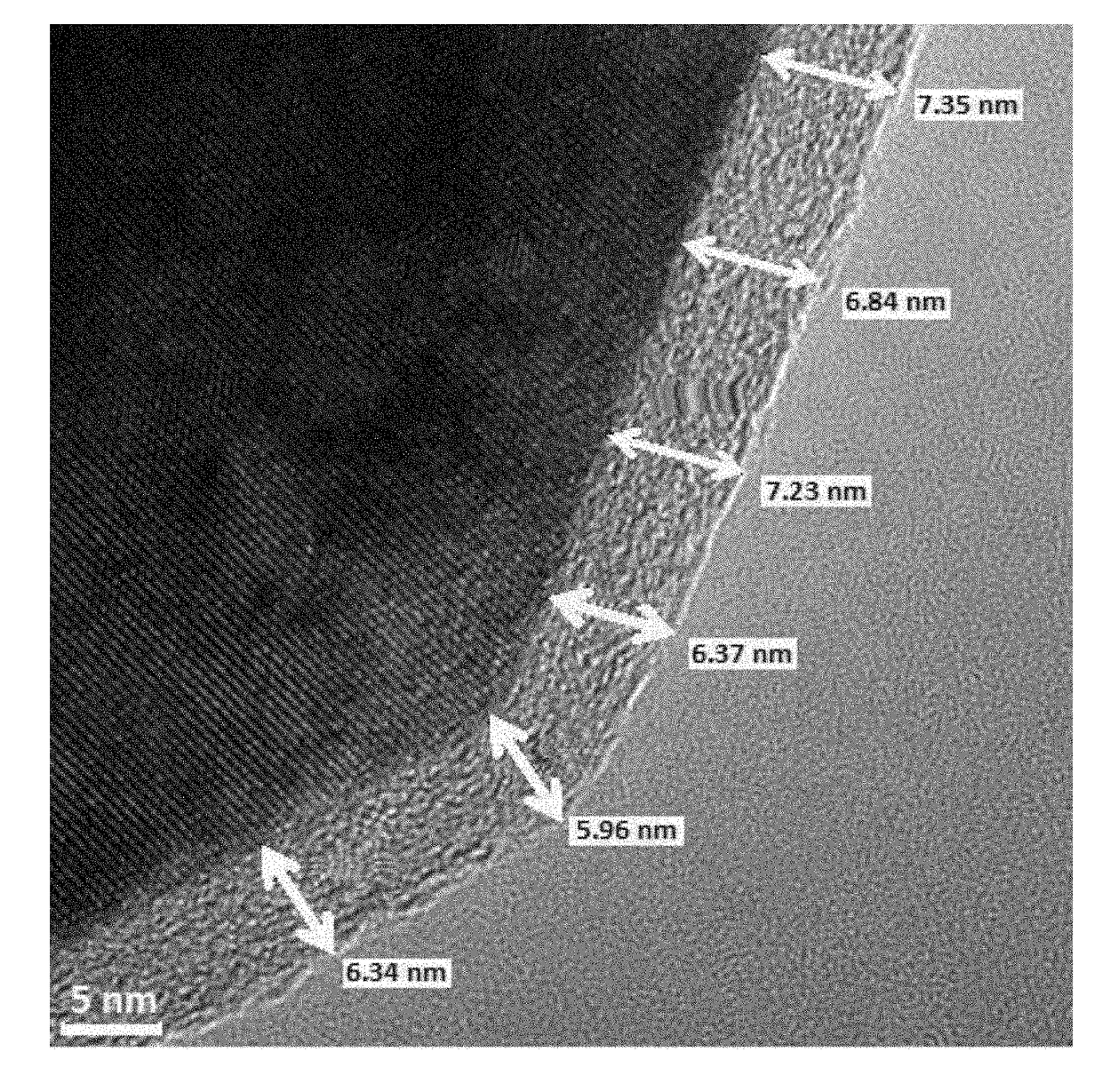
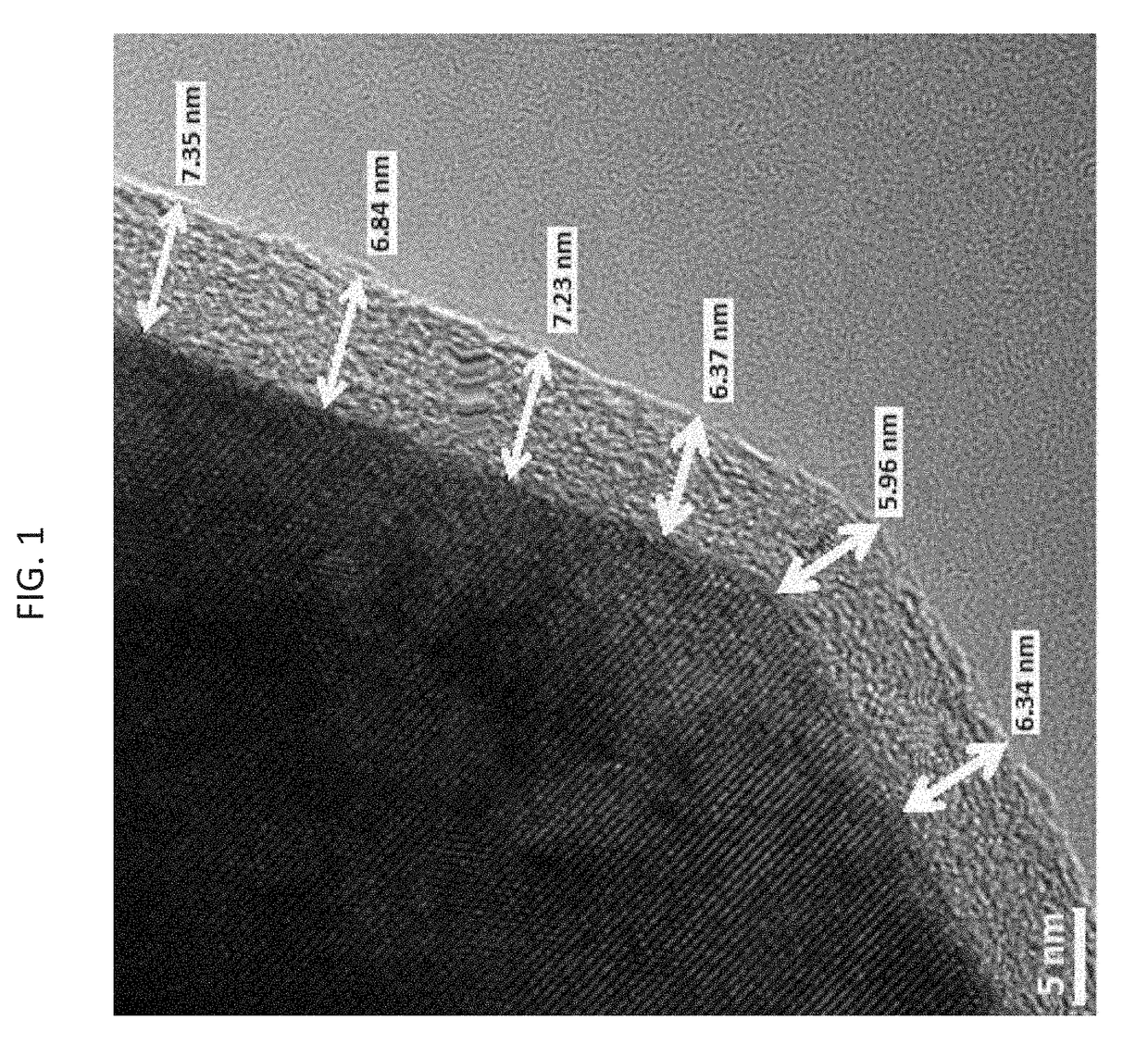
[0217]Below is an exemplary process for coating raw particulate lithium intercalation material which results in all-around coated particles, namely particles which are coated with a metal fluoride evenly and uniformly from all sides. The process does not alter the macroscopic structure of the particulate lithium intercalation material; hence, agglomerates and fused-together particles are treated as an individual entity with respect to their coated surface.
[0218]Powder coating by ALD became possible by a uniquely developed fluidized bed reactors (FBR). In such FBR reactor, the powder particles are floated in the chamber by means of a flow of an inert gas (i.e., dry nitrogen) jetted towards the sample from below. The gas jet is effected in order to move the particles with respect to themselves just before the precursors are introduced into the chamber.
[0219]Materials and Methods:
[0220]The active spinel-type cathode material, LiMn1.5Ni0.5O4 (LMNO...
example 2
Performance of Spinel-Type Cathode Material Coated with MgF2
[0239]Materials and Methods:
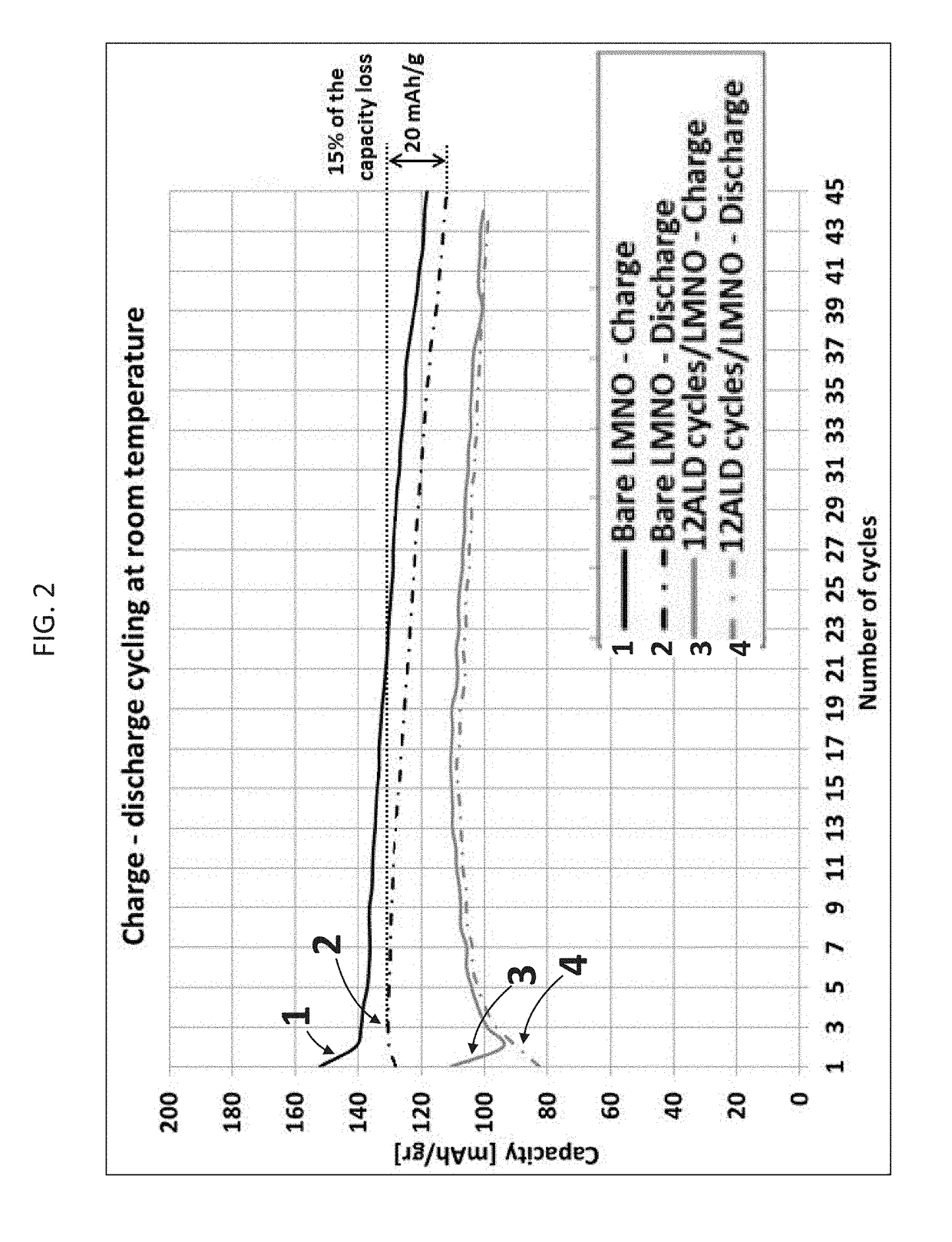
[0240]A lithium intercalation cathode was prepared using the MgF2-coated LMNO particles, prepared as described hereinabove and a conductive carbon black as an additive for LIB, and a resin binder.
[0241]Briefly, a slurry of the coated LMNO particles was prepared by mixing of 80 wt. % coated LMNO particles, 10 wt. % C-Nergy™ Super C45 (TIMCAL LTD, Bodio, Switzerland), 10 wt. % Kynar® PVDF resin (Arkema S.A., France) and N-methyl-2-pyrrolidone (NMP) as a solvent. The slurry was prepared by overnight component stirring using a magnetic stirrer, and was visually uniform before use. Thereafter the cathode sheet was prepared by casting the slurry on a top of aluminum foil current collector with doctor blade, followed by drying and thermo-treatment.
[0242]Discs of ½ inch in diameter were cut out from the above-described cathode sheet and assembled into T-type cells (Entegris, Inc., Billerica, Mass., USA)...
example 3
Electrolyte Effect on Cathode Material Coated with MgF2
[0250]The following experimental procedure was used to determine the level of leakage of elements from a lithium intercalation material to an electrolyte when exposed to the electrolyte under certain working conditions.
[0251]Materials and Methods:
[0252]The electrolyte effect on examples of particulate lithium intercalation material, LiMn1.5Ni0.5O4, (MNS) and LiNi1 / 3Mn1 / 3Co1 / 3O2 (NMC) powder, uncoated or coated with 6 or 12 atomic periods of MgF2, according to some embodiments of the present invention, was tested by analyzing the chemical composition of the electrolyte taken from cells, as described hereinabove, after the cells exhibited no change in the charge / discharge capacity (used-up cells).
[0253]Electrolyte samples were taken from each cell (0.2 ml) and mixed with 10 ml of distilled H2O and analyzed by inductively coupled plasma mass spectrometry (ICP-MS). The reference sample was the original electrolyte exposed to the pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com