Application of recombinant immunoregulatory protein of ganoderma lucidum in preparation of drug for treating focal cerebral ischemia
a technology of immunoregulatory protein and ganoderma lucidum, which is applied in the field of biological pharmacy, can solve the problems of serious threat to the health and life of people, and achieve the effect of significant therapeutic effect on the focal cerebral ischemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first example
Therapeutic Effect of rLZ-8 on Neurological Function Injury
[0016]1. Experimental Material
[0017]Rats, having a weight of 250-300 g, half males and half females, were provided by Laboratory Animal Center of Jilin University. GM-1 was provided by Beijing Four-ring Pharmaceutical Co., Ltd. Surgical instruments.
[0018]2. Experimental Method
[0019]Preparing rat focal cerebral ischemia models through a modified Zea Longa method; grading the rats which were fully conscious after operation according to a modified NSS grading standard (16-point system); arranging a sham operation group, a model group, a positive drug control group, a rLZ-8 high-dosage group (70 μg·kg−1), a rLZ-8 middle-dosage group (35 μg·kg−1) and a rLZ-8 low-dosage group (17.5 μg·kg−1); and injecting intraperitoneally, twice a day, wherein the same volume of physiological saline was injected into the sham operation group and the model group, for consecutive 7 days. A positive drug in the positive drug control group was the GM...
second example
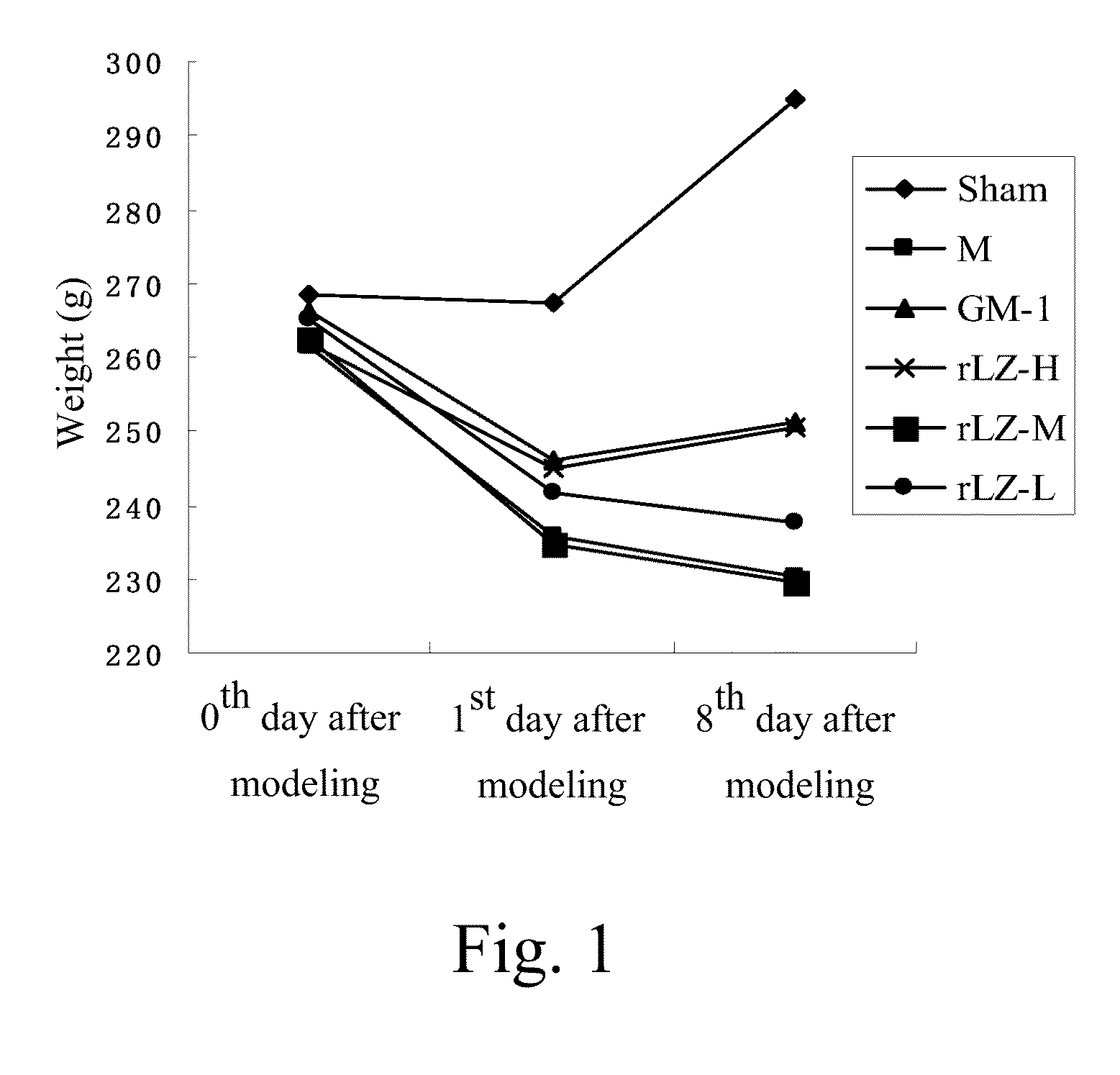
Influence of rLZ-8 on Weights of Rats
[0022]1. Experimental Method
[0023]Weighing rats before modeling; preparing rat focal cerebral ischemia models through a modified Zea Longa method; weighing the rats on the first postoperative day; and weighing the rats after consecutively administrating a drug for 7 days.
[0024]2. Experimental Results
[0025]As shown in Table 2 and FIG. 1, before modeling, weights of the rats of each group had no significant difference; after modeling, the weights of the rats of each group were significantly decreased; and after consecutively administrating the drug for 7 days, the weights of the rats of the rLZ-8 high-dosage group were increased, illustrating that the rLZ-8 is able to reduce an injury caused by cerebral ischemia.
TABLE 2Changes of weights of rats of each group (x± s)0th day after1st day after8th day afterGroupmodelingmodelingmodelingSham268.33 ± 13.87267.17 ± 13.73295.00 ± 24.22 M261.50 ± 16.57 235.67 ± 18.97** 230.17 ± 26.49**GM-1266.17 ± 21.21246....
third example
HE Staining Experiment
[0026]1. Experimental Method
[0027]Perfusing and fixing: anesthetizing a rat by chloral hydrate and fixing the rat in a supine position; processing the rat with thoracotomy to expose a heart; inserting a perfusing needle into an aorta through an apex of a left ventricle; firstly perfusing the rat quickly by physiological saline, and meanwhile, cutting off a right auricle and clamping the aorta by a hemostatic forceps; when no blood was in an effluent liquid, quickly perfusing the rat by fixative, and stopping perfusing when a body of the rat became stiff; cutting off a head to remove out a brain tissue; preparing a coronary brain section through a rat brain section mold; fixing the coronary brain section by formalin; selecting a brain section (the third brain section) which was horizontal with an optic chiasma; embedding the brain section which was horizontal with an optic chiasma by conventional paraffin and sectioning.
[0028]HE staining: (1) dewaxing the embedd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com