Methods and systems for processing cellulosic biomass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Distillation of Total Reactor Contents
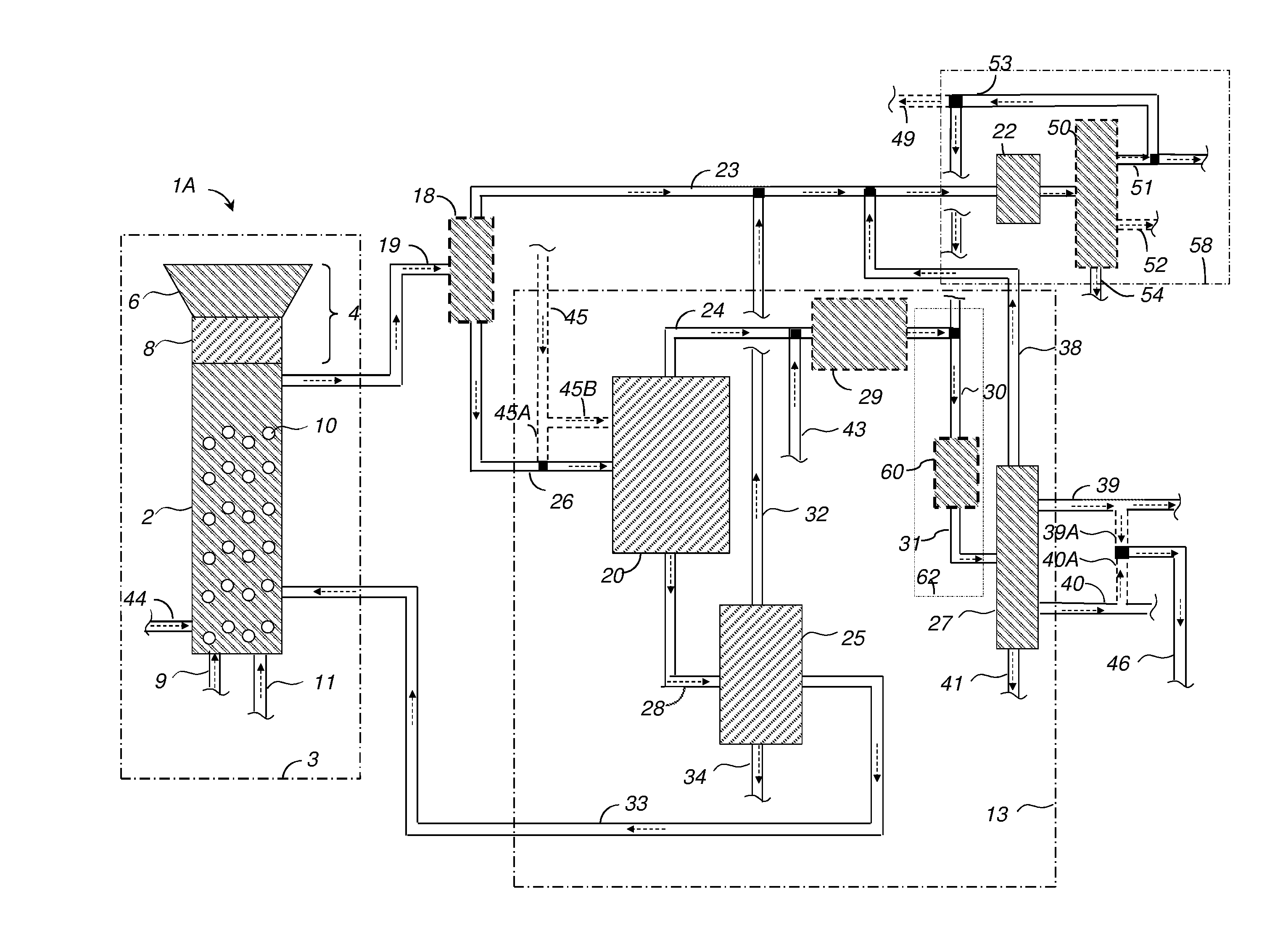
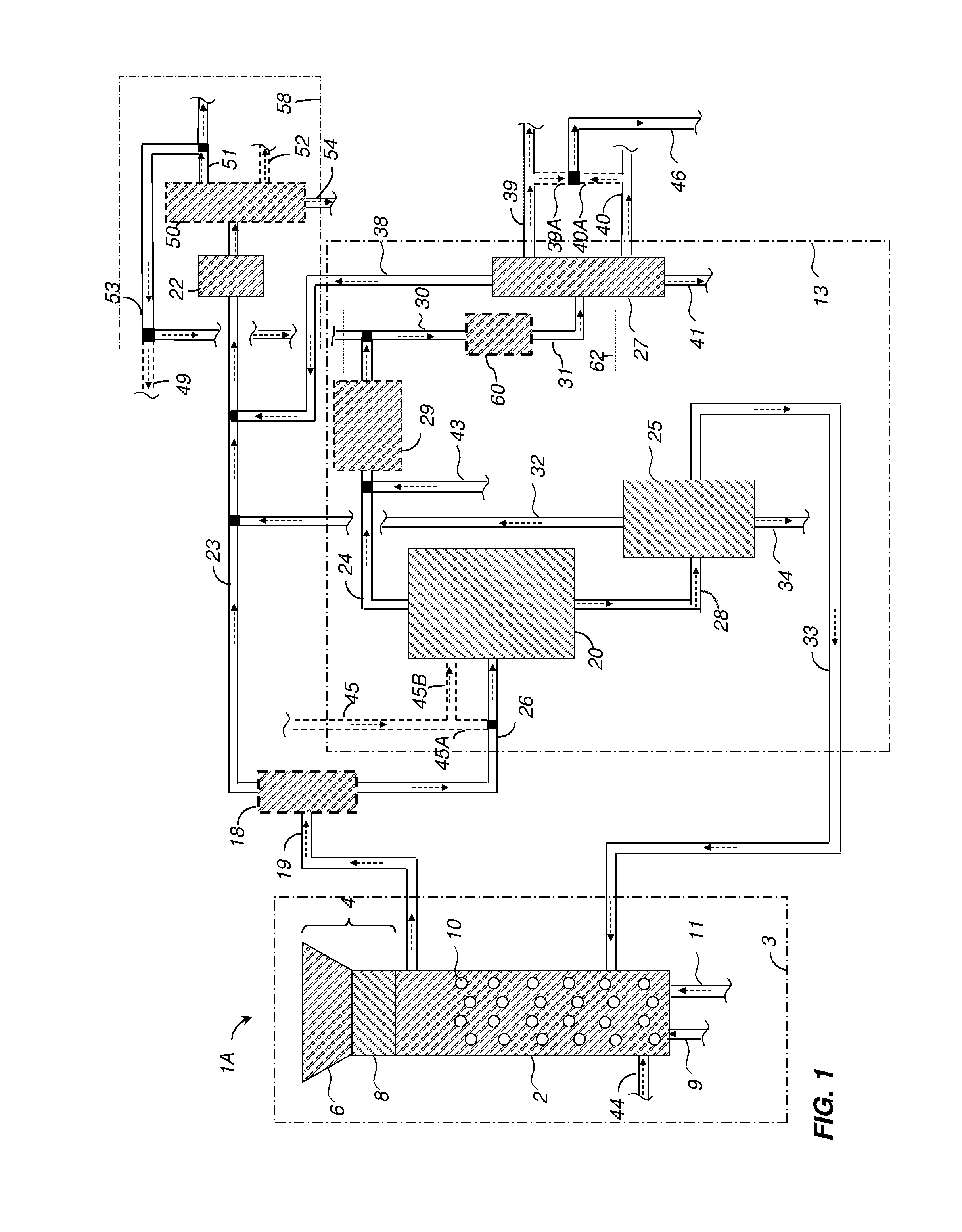
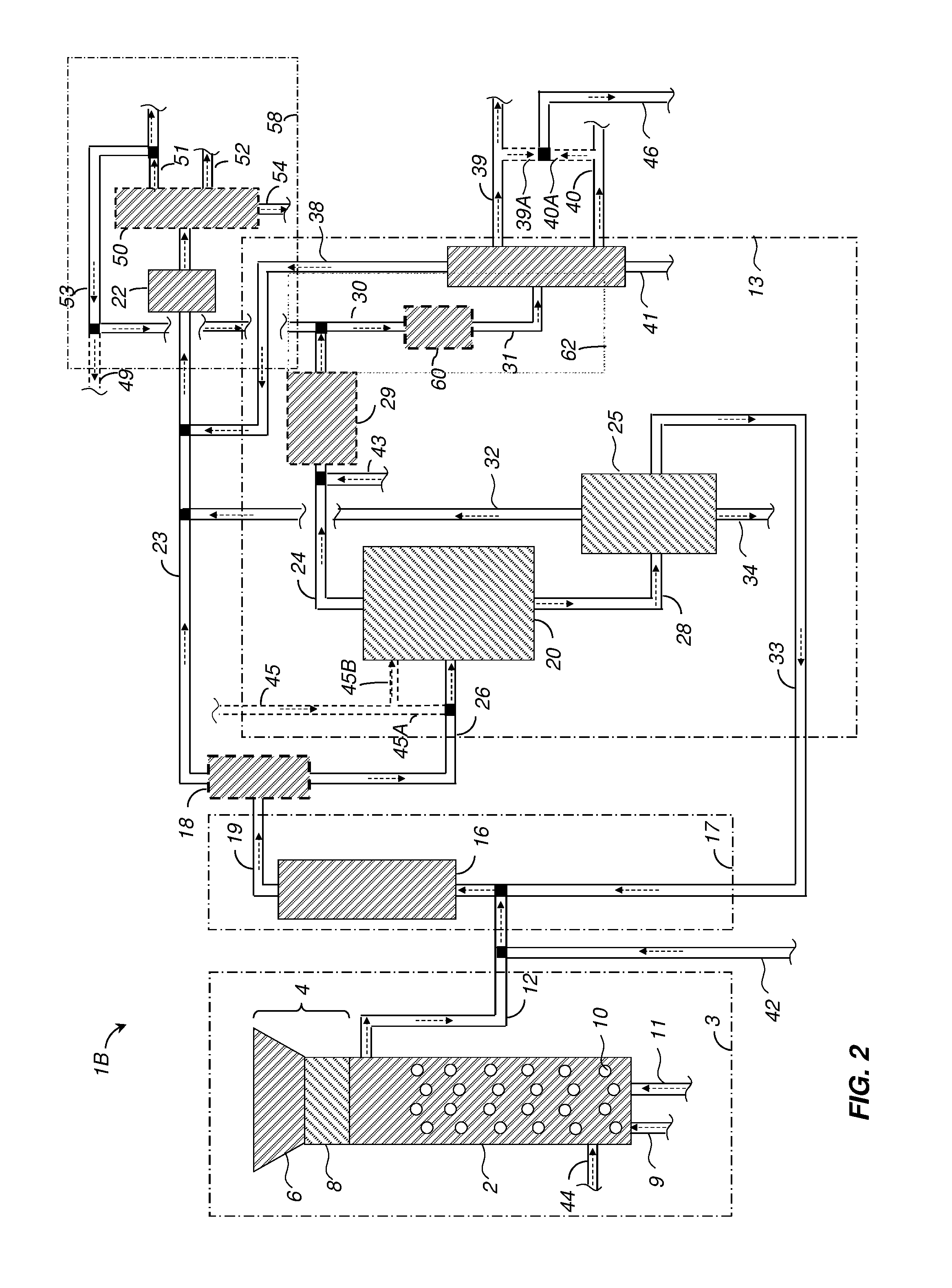
[0157]A 450-ml Parr reactor was charged with 20.02 grams of 2-methoxy-4-propylphenol (MPP), 190.01 grams of deionized water, 0.4192 grams of potassium hydroxide buffer, and 7.2522 grams of nickel-oxide promoted cobalt molybdate catalyst (DC-2534, containing 1-10% cobalt oxide and molybdenum trioxide (up to 30 wt %) on alumina, and less than 2% nickel), obtained from Criterion Catalyst & Technologies L.P., and sulfided by the method described in US2010 / 0236988 Example 5.
[0158]The reactor was then charged with 14.02 grams of southern pine mini-chips (10% moisture), of nominal size 3×5×5 mm in dimension, before pressuring with 52 bar of hydrogen, and heating to 190° C. for 1 hours, then ramping to 245° C. for 2.5 hours.
[0159]After reaction, the reactor was cooled and depressured. A nominal 14 grams of wood chips were added, followed by repressuring with 52 bar H2, and conducting a second reaction cycle using the same heating profile. The sequence w...
example 2
Separation by Extraction Followed by Distillation of Separate Phases
[0169]Example 1 was repeated using 5.0035 grams of Raney™ cobalt catalyst (WR Grace 2724), and a solvent comprising of 21.1 grams of 2,6-xylenol as the phenolic solvent, solubilized first into 7.00 grams of methylisobutylcarbinol, and added together with 182.01 grams of deionized water to the 450-ml reactor. Reaction cycles entailed 1 hour at 160° C., followed by 1 hour at 190° C., followed by 3 hours at 240° C. Six cycles of wood addition were effected, followed by heating overnight at 270° C. under 52 bar of H2 to complete the conversion of intermediates.
[0170]At the end of the reaction sequence, 87.03 grams of toluene (simulated aromatics gasoline product) were added to the reactor, and the reactor was stirred at 150° C. for 2 hours to effect extraction. Stirring was then stopped, and the reactor was allowed to cool. An upper toluene-rich phase of 127.39 grams was decanted, followed by 220.65 grams of a lower aqu...
example 3
Miscibilizing Solvent Addition
[0181]A 75-ml Parr5000 reactor was charged with 3.01 grams of xylenol (2,6-dimethylphenol) and 21.07 grams of deionize water, 0.107 grams of potassium carbonate buffer, and 0.301 grams of Raney Cobalt 2724 catalyst (WR Grace). 2.0 grams of ground Southern pine wood (nominal 10% moisture) were charged. The reactor was pressured to 52 bar with hydrogen, and heated to 190° C. for 1 hour, followed by heating to 240° C. for 4 hours.
[0182]Three more cycles of nominal 2.0 grams of wood addition were completed, before a final cycle entailing pressuring to 35 bar with hydrogen, and heating to 270° C. for 15 hours to extend the extent pf conversion of hydrodeoxygenation reactions.
[0183]At the end of the conversion cycle, the reactor was cooled and depressured. 44.3 grams of acetone were added to dissolve all reactor contents. Filtration yielded 0.3 grams of recovered catalyst, as evidenced by magnetic attraction, with no visible organic solids present, and no dep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Condensation enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com