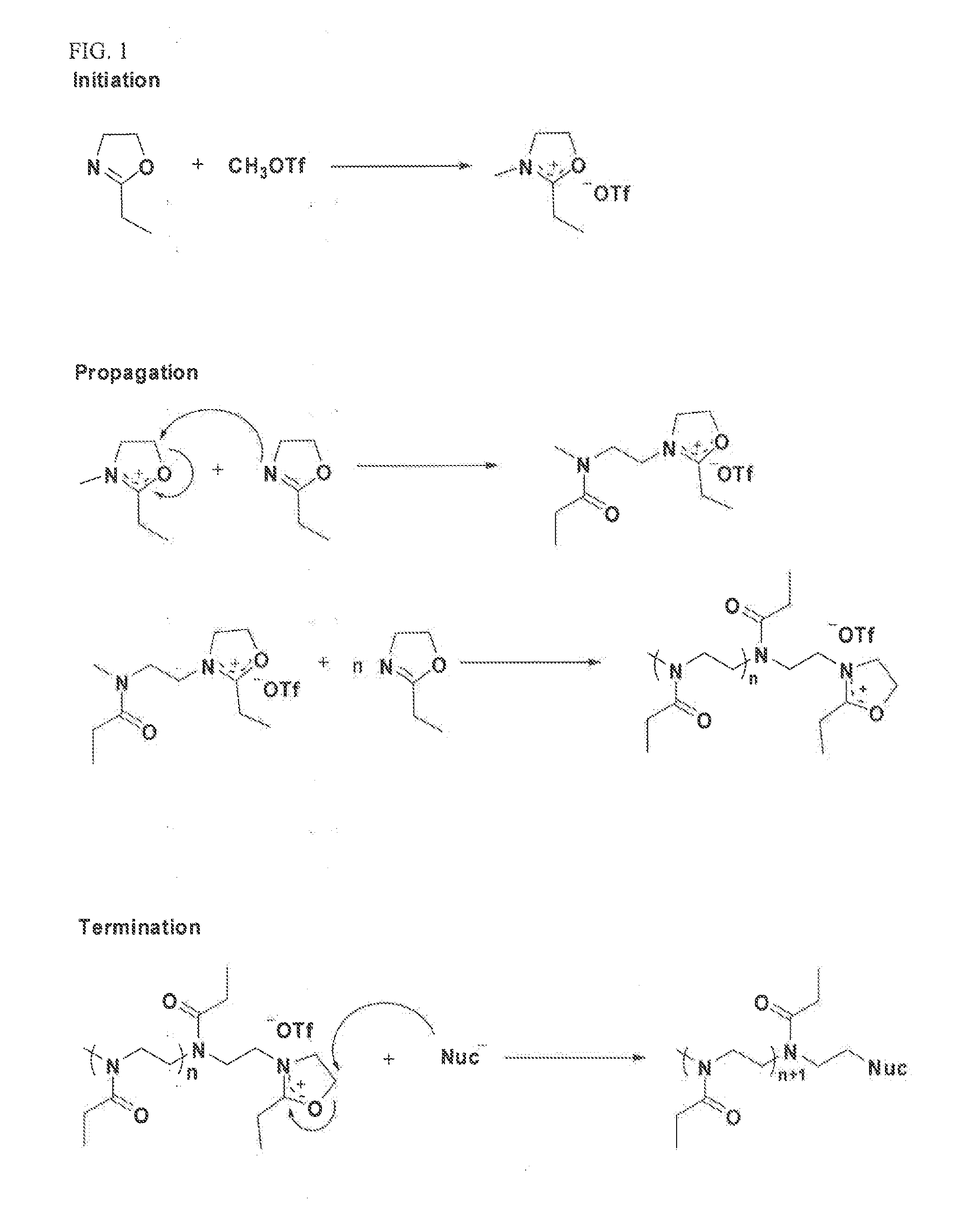
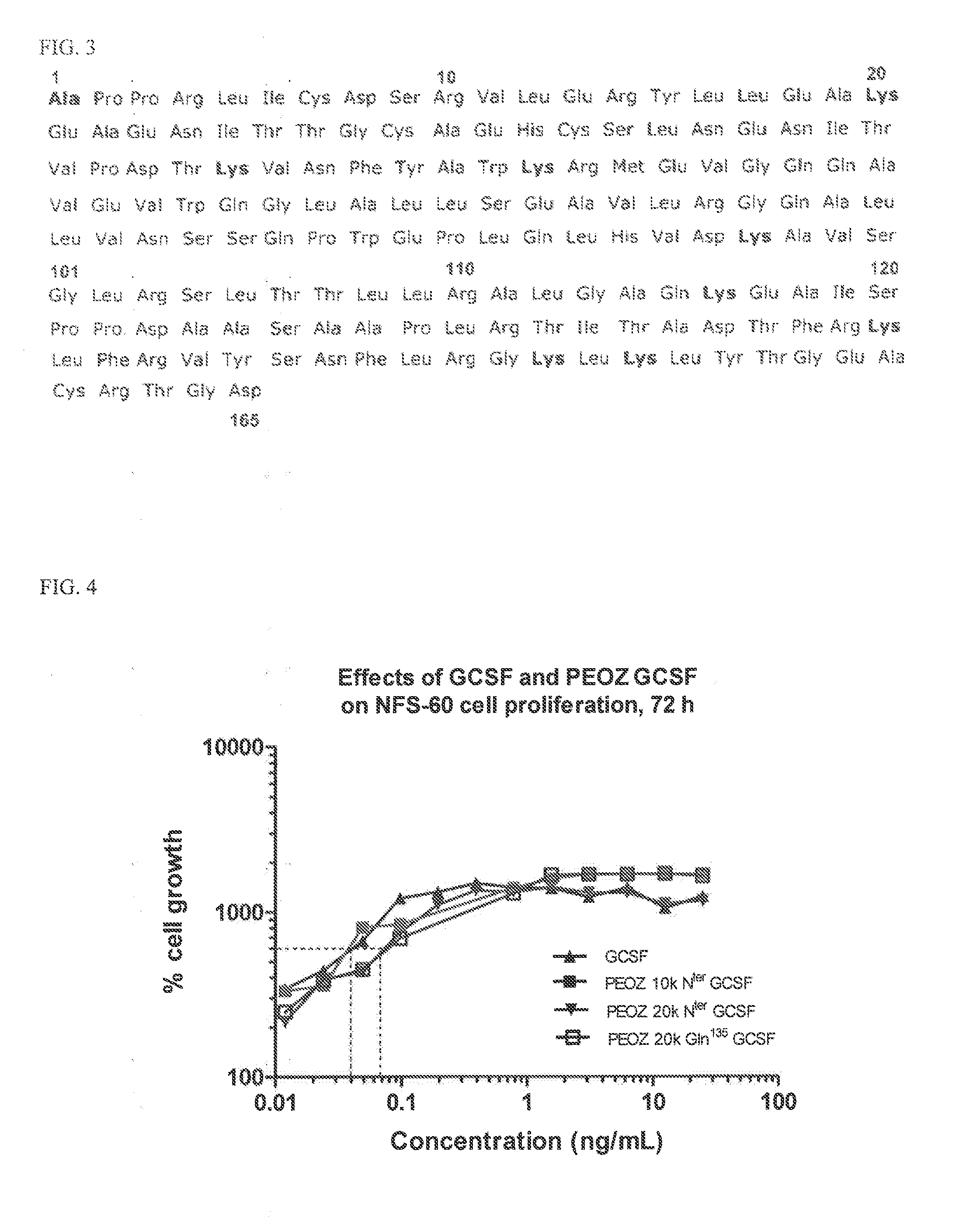
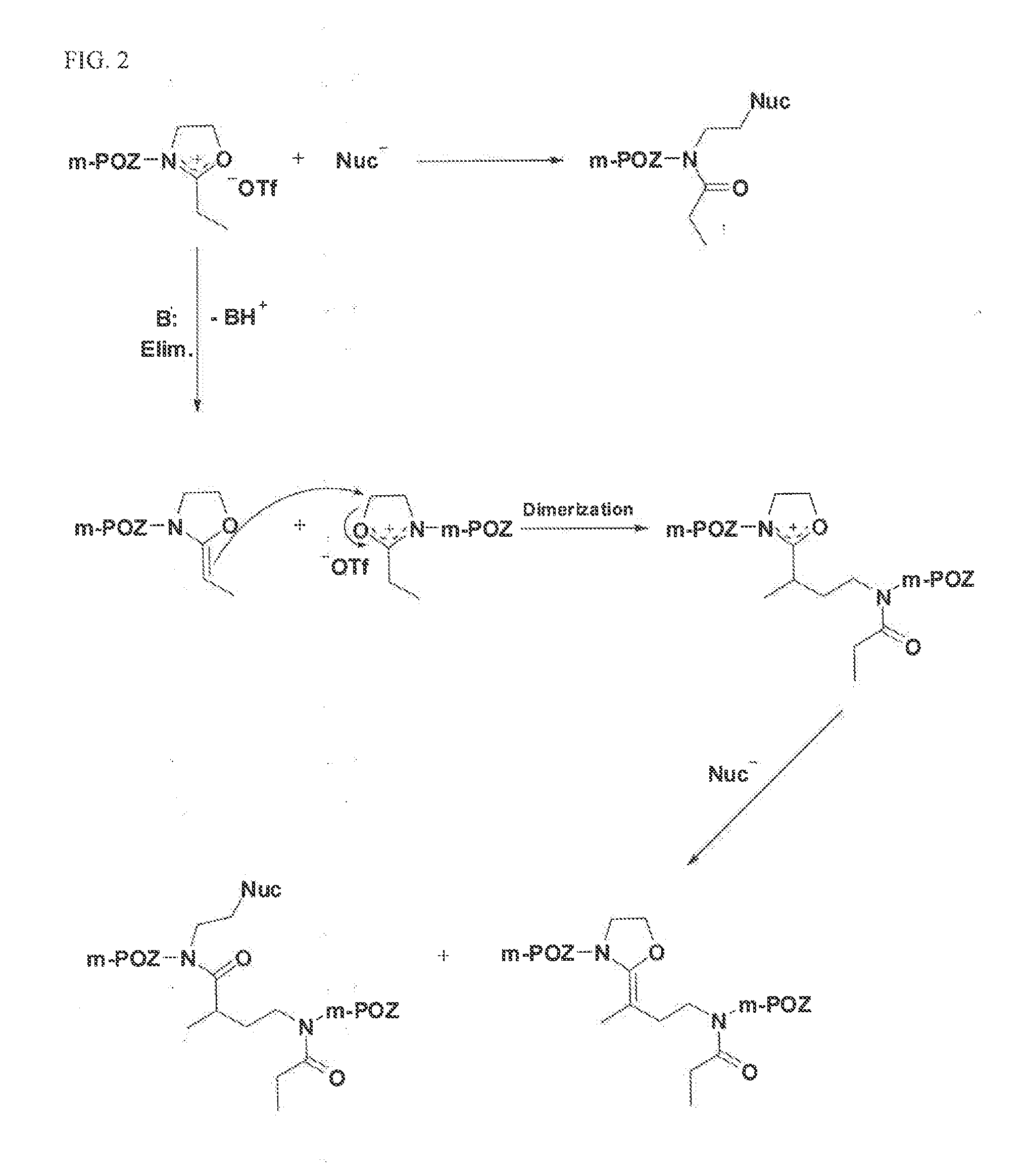Activated Polyoxazolines and Conjugates and Compositions Comprising the Same
a technology of activated polyoxazolines and conjugates, which is applied in the field of polyoxazoline polymers and polyoxazoline derivatives, can solve the problems of low work done on the activation of poz for coupling to target molecules, high polydispersity, and limitations of each of the previously described poz derivatives, so as to improve in-vivo stability, reduce renal clearance, and increase protein effective size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 20 kDa H-PEOZ-Hydrazid
[0201]
[0202]The synthesis of 20 kDa polyoxazoline succimidyl thiopropionate has been described (H-PEOZ-T-SPA 20K, GFC shows 83% of —O-Su; GPC showed a Mn=18,243 Da, PDI=1.055, Mp=19,973 Da; and MALDI-TOF showed a Mn=20,972 Da). Hydrazine monohydrate, NH2NH2.H2O, 98% was from Aldrich, FW 50.06, d 1.032.
[0203]H-PEOZ-T-SPA 20K (2.0 gm, 9.1240×10−5 mol, 1 equiv.) was first dissolved in anhydrous dichloromethane (90 mL) and this solution was transferred into an addition funnel. In a 250 mL round bottom flask, hydrazine monohydrate (452 μL, 9.1240×10−3 mol, 100 equiv.) was dissolved in anhydrous dichloromethane (5 mL). Under an argon atmosphere, the H-PEOZ-T-SPA solution in dichloromethane was added to the hydrazine solution drop wise over one hour with rapid stirring. This solution was stirred overnight at ambient temperature and in an argon atmosphere. The white precipitate that was formed in the reaction mixture was filtered out and the filtrate was...
example 2
Conjugation of Heparin by 20 kDa H-PEOZ-Hydrazide Through Carbohydrate Reducing Terminus
[0204]A 50 mg / mL solution of 20 kDa H-PEOZ-Hydrazide (2,222 μL, 5.5556×10−6 mole, 5 equiv.) was added to heparin sodium salt (Grade 1-A, purchased from Sigma-Aldrich, MW 17000-19000 Da, 20 mg, 1.1111×10−6 mole, 1 equiv.) in pH 3.0 acetic acid solution. The solution was allowed to stir at room temperature for 10 minutes. The solution pH was adjusted to 3.0 by adding 0.1 N HCl acid. To this solution, freshly prepared 1 M NaBH3CN in deionized water (111.1 μL, 1.1111×10−4 mole, 100 equiv) was added. The solution was stirred at room temperature for 10 minutes, and then incubated at 37° C. for overnight. The reaction mixture was analyzed by GFC using a BioSEP-SEC-S 4000 column. GFC showed the formation of H-PEOZ-heparin conjugate (data not shown).
example 3
Conjugation of Dynorphin A (1-13) at C-Terminus by 5 kDa M-PEOZ-Hydrazide using EDC as Coupling Agent
[0205]
[0206]Dynorphin A (dynorphin 1-13) (Bachem, 0.55 mg, 2.2133×10−7 mol, 1 equiv.) was dissolved in 250 μL of 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer at pH 3.0. A 109.5 μL aliquot of 50 mg / mL 5 kDa M-PEOZ-Hydrazide 5K (5.5 mg, 1.1066×10−6 mol, 5 equiv) in 50 mM MES buffer at pH 3.0 was prepared and filtered through a 0.2 μm syringe filter. This 5 kDa M-PEOZ-Hydrazide solution was then added to the dynorphin A solution. The solution pH was adjusted to 3.0 by drop by drop addition of a 50 mM HCl acid solution. A solution of N-(3-Dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride (EDC, purchased from Fluka) in deionized water (31 μL, 100 mg / mL, 1.6097×10−5 mol, 20 equiv.) was added into the mixture. The solution pH was adjusted to 3.0 by slowly adding 50 mM HCl. The solution was allowed to stir at room temperature for 3 hours. The reaction mixture was analyzed by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com