Oligomers
a technology of oligomers and oligomers, applied in the field of oligomers, can solve the problems of undesirable immune responses and long-term sustainability difficulties, and achieve the effects of reducing specificity of binding to the target site, excellent safety profiles, and long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Bioinformatics Analysis of the Myostatin Gene to Design AOs Reagents.
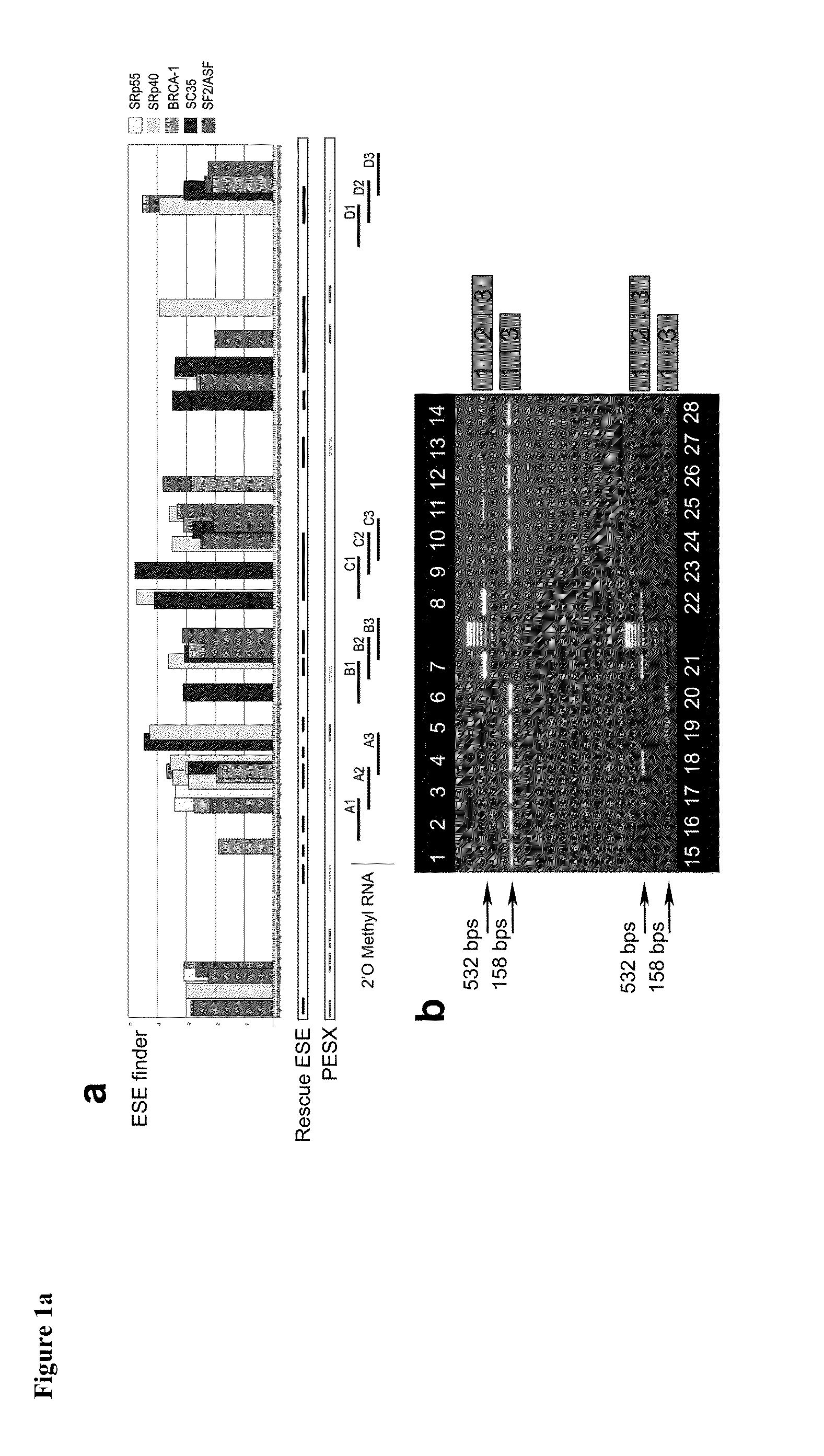
[0041]Three different bioinformatics algorithms namely ESE Finder, PESX, and Rescue ESE were used to design antisense reagents. Results from the three algorithms were merged to define ESE sites and used to identify the regions of the myostatin exon 2, which are expected to be optimal targets for exon skipping antisense reagents. A set of 12 antisense reagents of 2'O-methyl RNA (2'OMePS) chemistry were designed to target four different ESE-rich regions of exon 2 of myostatin (FIG. 1a).
AO Reagents.
[0042]The 12 2'OMePS oligomers tested were obtained from Eurogentec (SA, Seraing, Belgium). The sequences of the 2'OMePS are as follows:
GDF8 / A1:TCCACAGTTGGGCTTTTACTGDF8 / A2:TCTGAGATATATCCACAGTTGDF8 / A3:TCTTGACGGGTCTGAGATATGDF8 / B1:TGATGAGTCTCAGGATTTGCGDF8 / B2:TTCATGGGTTTGATGAGTCTGDF8 / B3:TTGTACCGTCTTTCATGGGTGDF8 / C1:CAGAGATCGGATTCCAGTATGDF8 / C2:TGTCAAGTTTCAGAGATCGGGDF8 / C3:CCTGGGCTCATGTCAAGTTTGDF8 / D1:CTGGGAAGGT...
example 2
[0062]The 30-mer PMO AOs tested above (Mstn-A to Mstn-D) were designed to target the myostatin gene in mice. Therefore, these Mstn-A to Mstn-D sequences correspond (are complementary to) to the Genbank mouse myostatin cDNA / mRNA gene sequence. The corresponding sequences complementary to the Genbank human myostatin sequences are as follows with differences between the Genbank mouse and human underlined:
Hum Mstn A:TCTCGACGGGTCTCAAATATATCCATAGTTHum Mstn B:TGTACCGTCTTTCATAGGTTTGATGAGTCTHum Mstn C:CCTGGGTTCATGTCAAGTTTCAGAGATCGGHum Mstn D:CAGCCCATCTTCTCCTGGTCCTGGGAAGGT
[0063]The skipping efficiency of these AOs can be tested by transfection (leashed or unleashed: concentration between 50 and 500 nM) into cultured human myoblast cells (eg using a transfection reagent such as Lipofectamine2000™), and evaluation of skipped and unskipped mRNAs by electrophoretic densitometric analysis of RTPCR reaction products.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com