Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
114 results about "Increase muscle mass" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
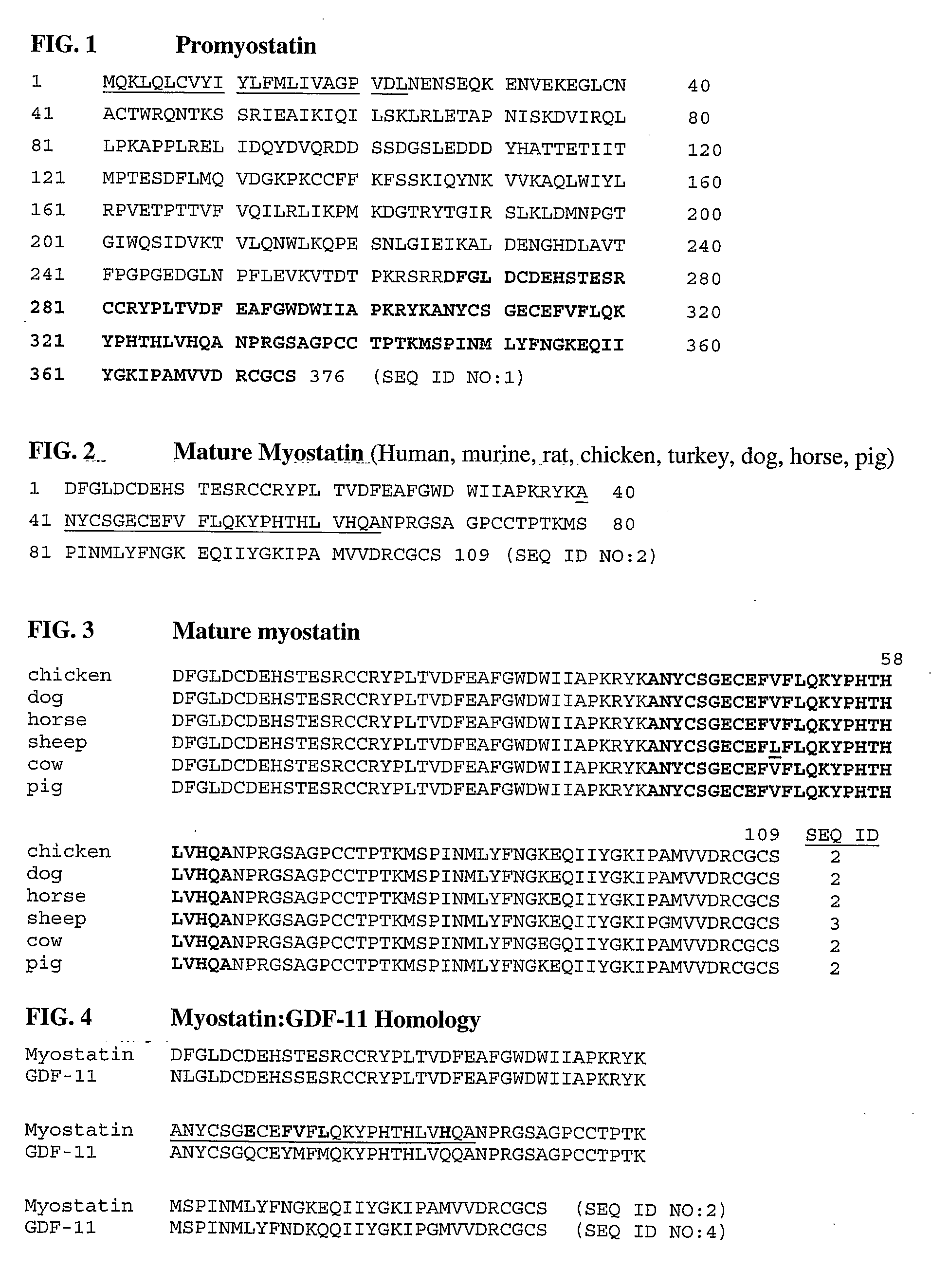
Avian follistatin product
InactiveUS20070275036A1Prevent muscle wastingAvoid wastingBiocidePeptide/protein ingredientsFowlAnimal science
Described herein are avian follistatin products, and methods for producing such products, in which such products are effective for a variety of conditions, including increasing muscle mass. Avian follistatin products described herein are packaged as dietary supplements or nutritional supplements are useful in muscle regeneration.
Owner:MYOS
Compositions and methods for increasing muscle mass and muscle strength by specifically antagonizing GDF8 and or Activin A
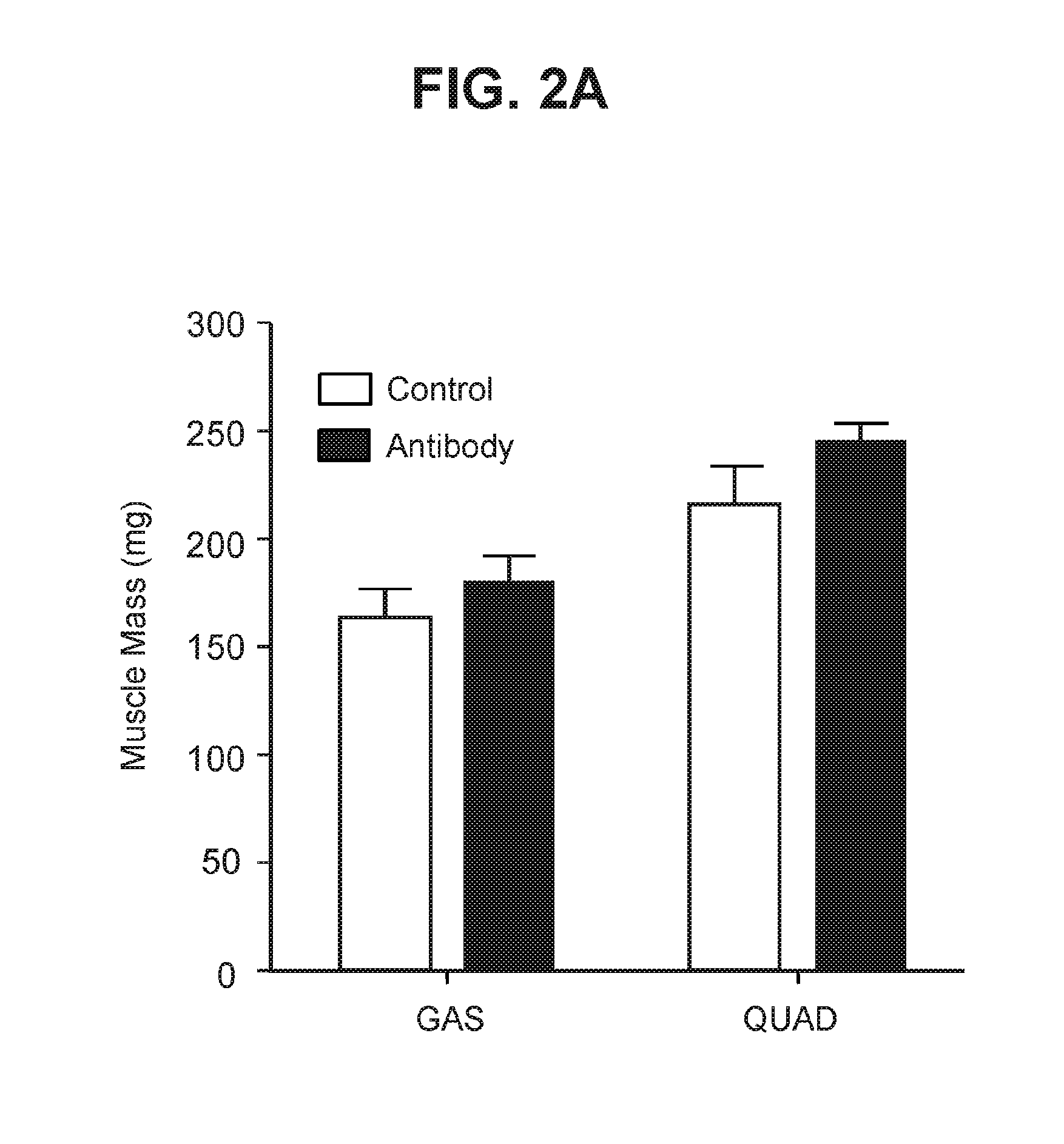
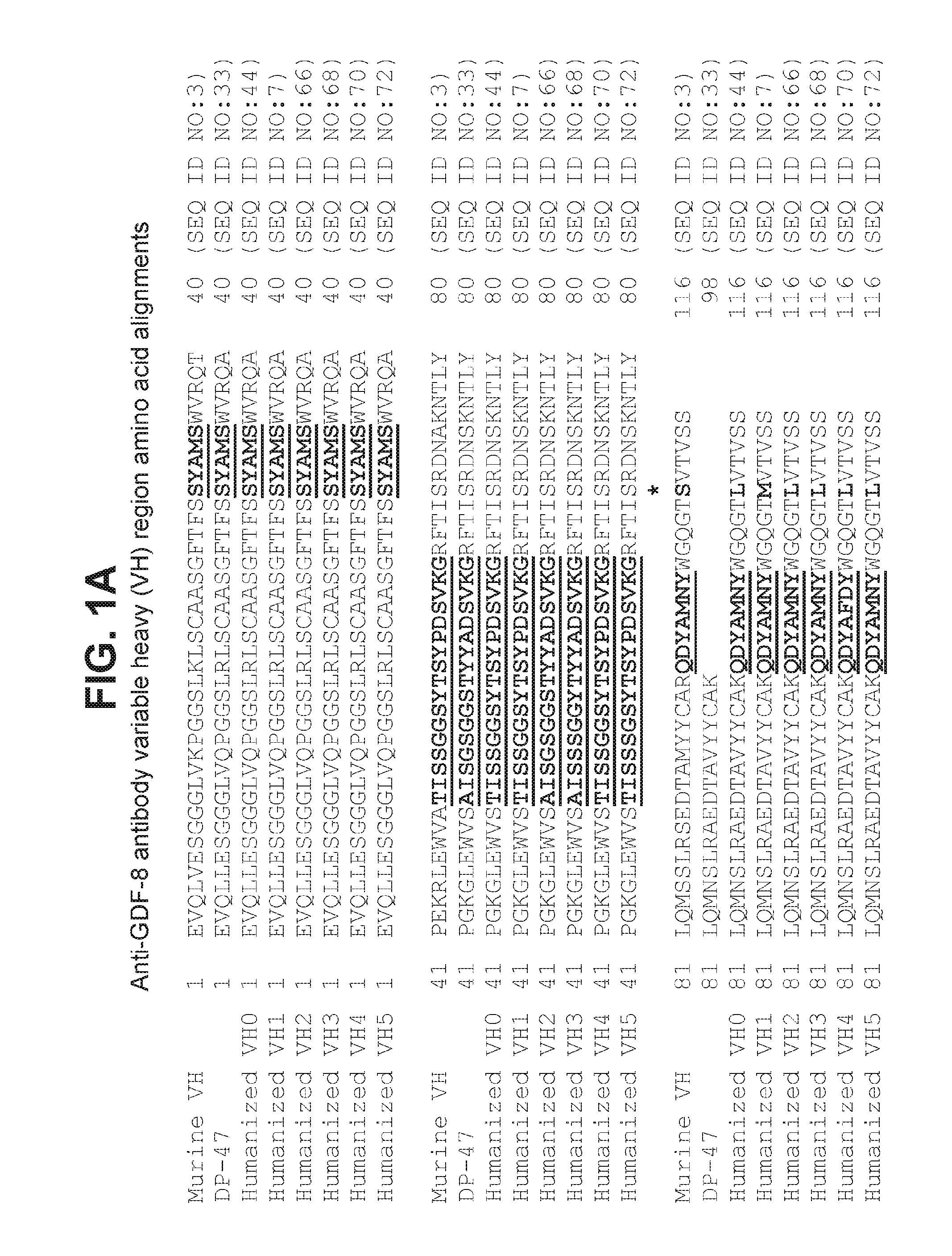
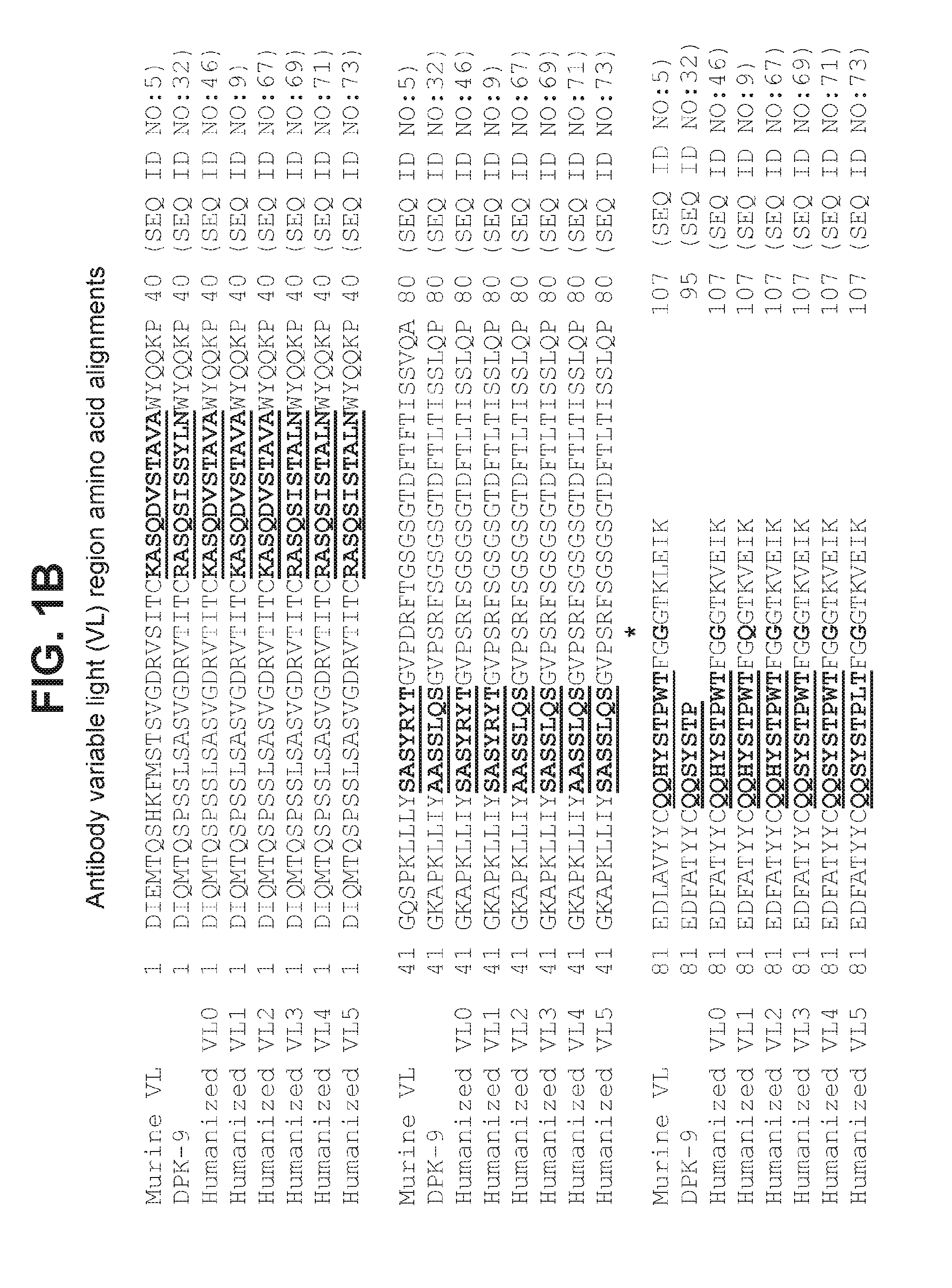
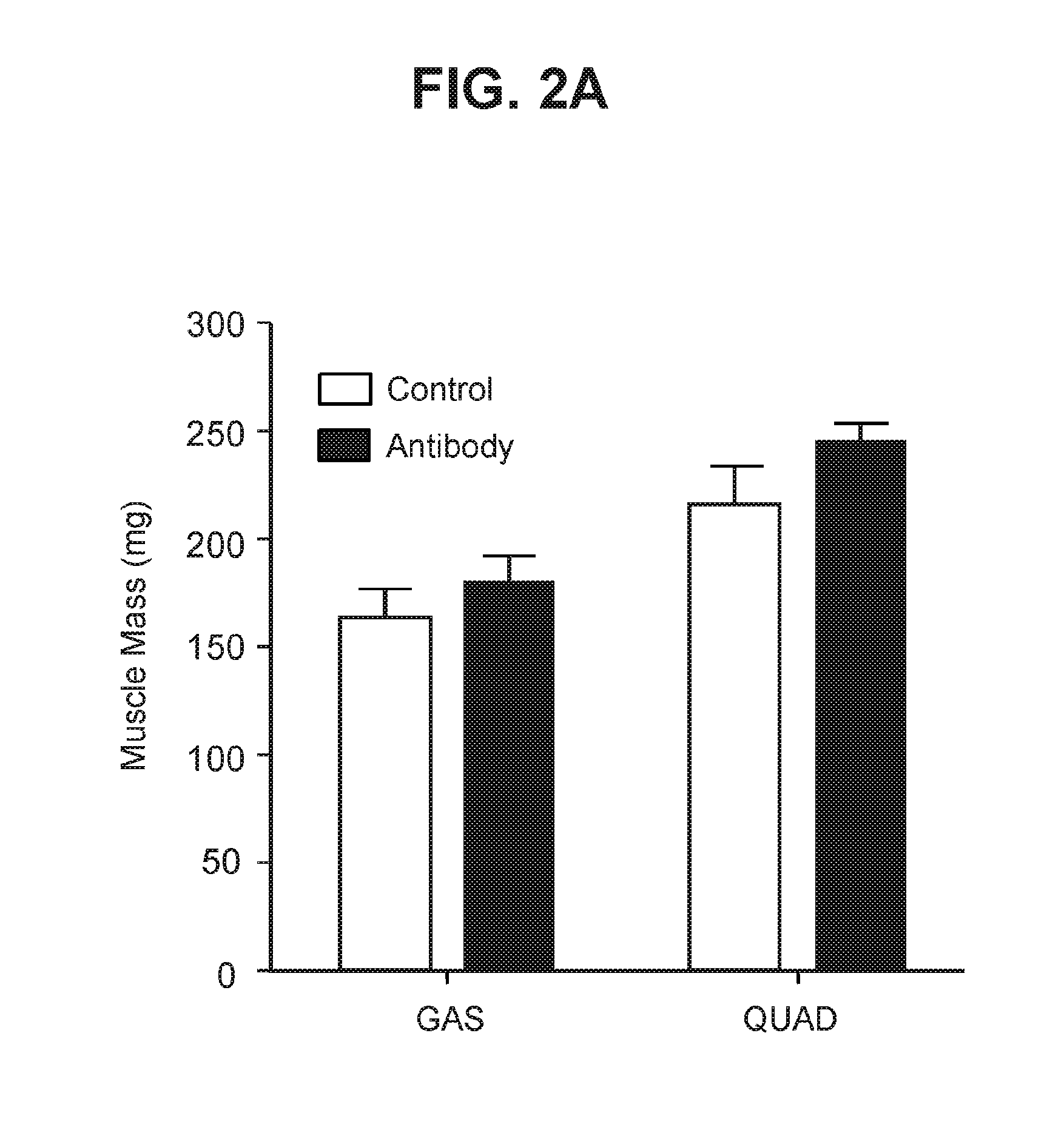
The present invention provides compositions and methods which involve specifically antagonizing GDF8 and Activin A. In certain embodiments, compositions are provided which comprise a GDF8-specific binding protein and an Activin A-specific binding protein. For example, the invention includes compositions comprising an anti-GDF8 antibody and an anti-Activin A antibody. In other embodiments, antigen-binding molecules are provided which comprise a GDF8-specific binding domain and an Activin A-specific binding domain. For example, the invention includes bispecific antibodies that bind GDF8 and Activin A. The compositions of the present invention are useful for the treatment of diseases and conditions characterized by reduced muscle mass or strength, as well as other conditions which are treatable by antagonizing GDF8 and / or Activin A activity.
Owner:REGENERON PHARM INC
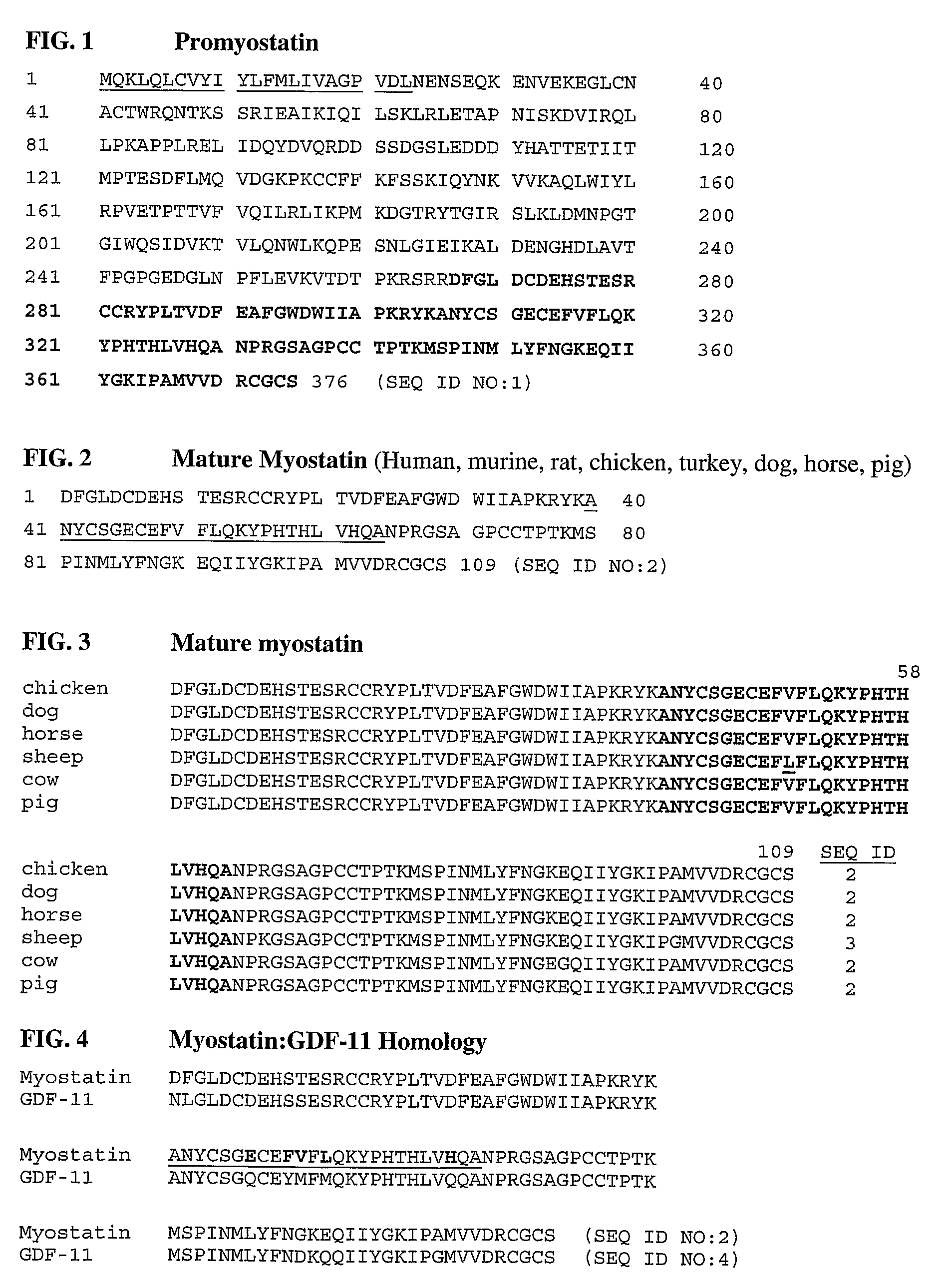
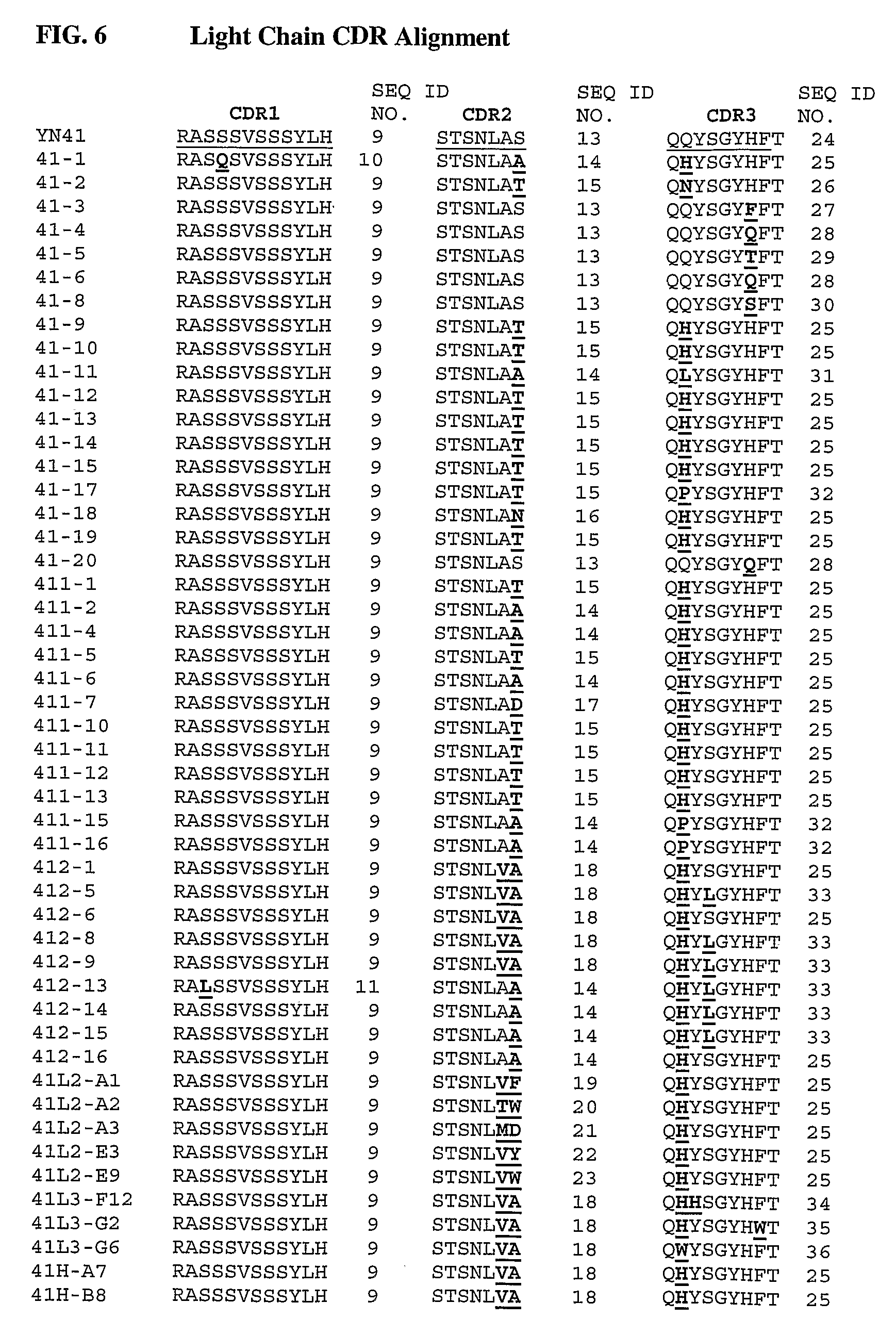
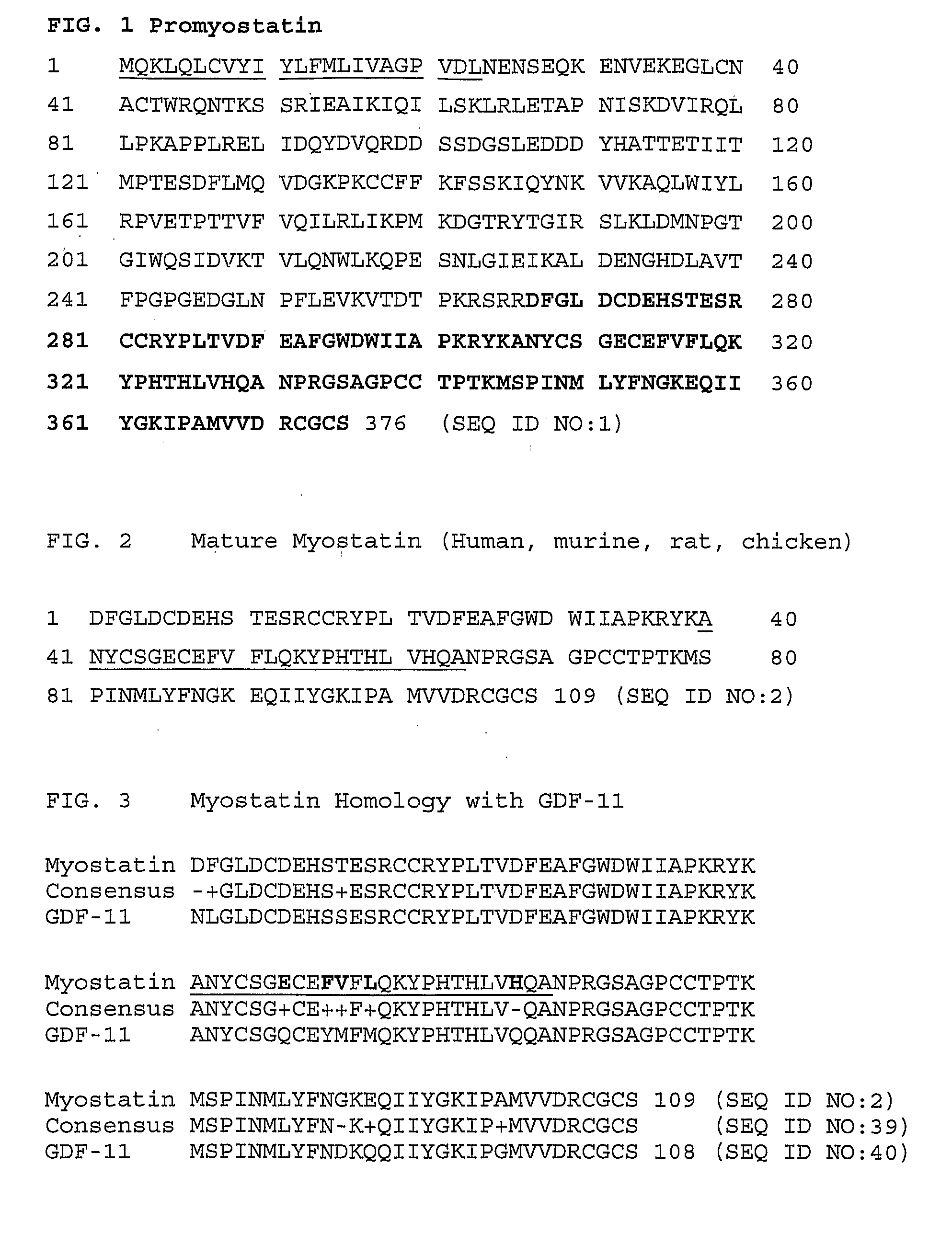
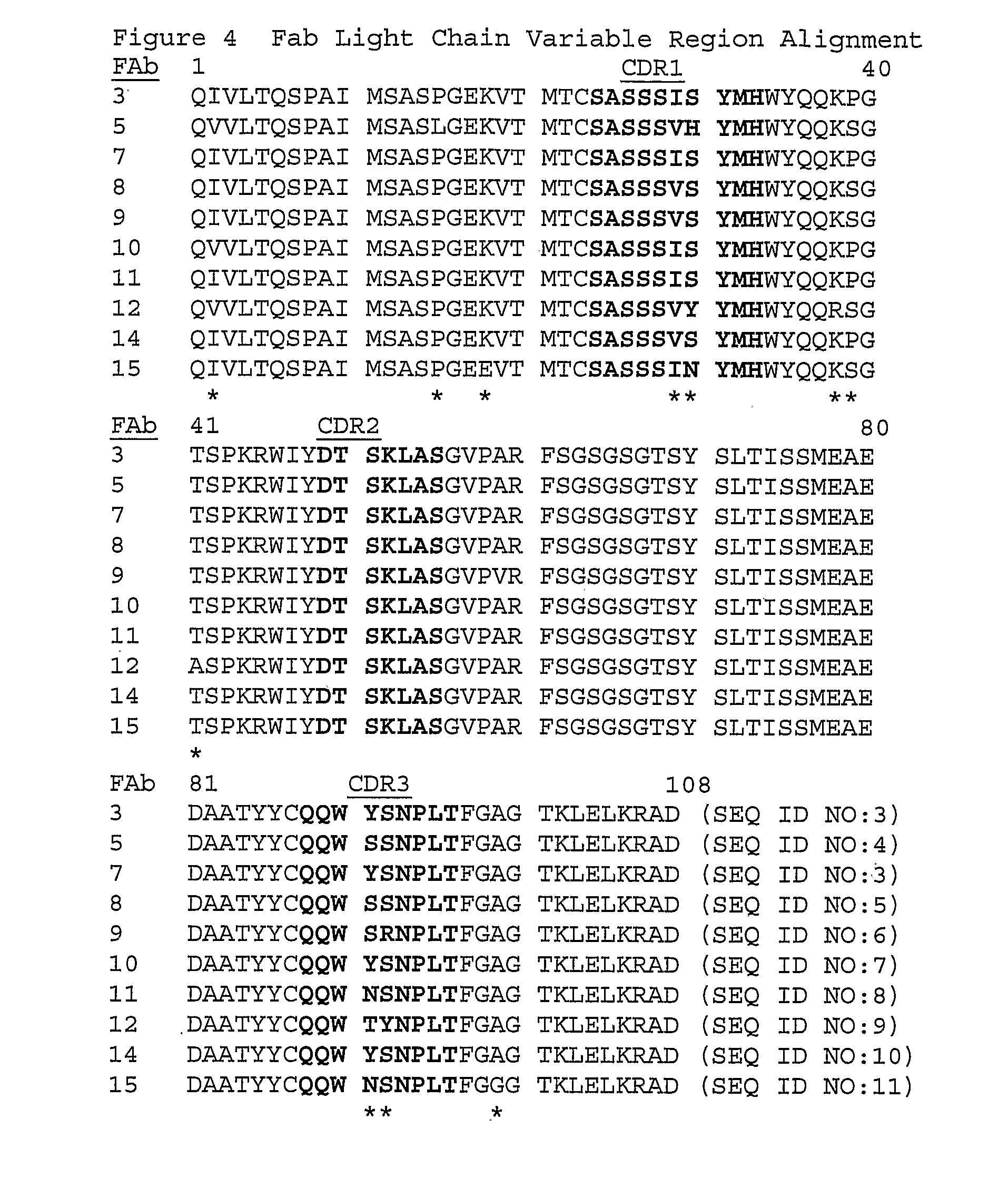
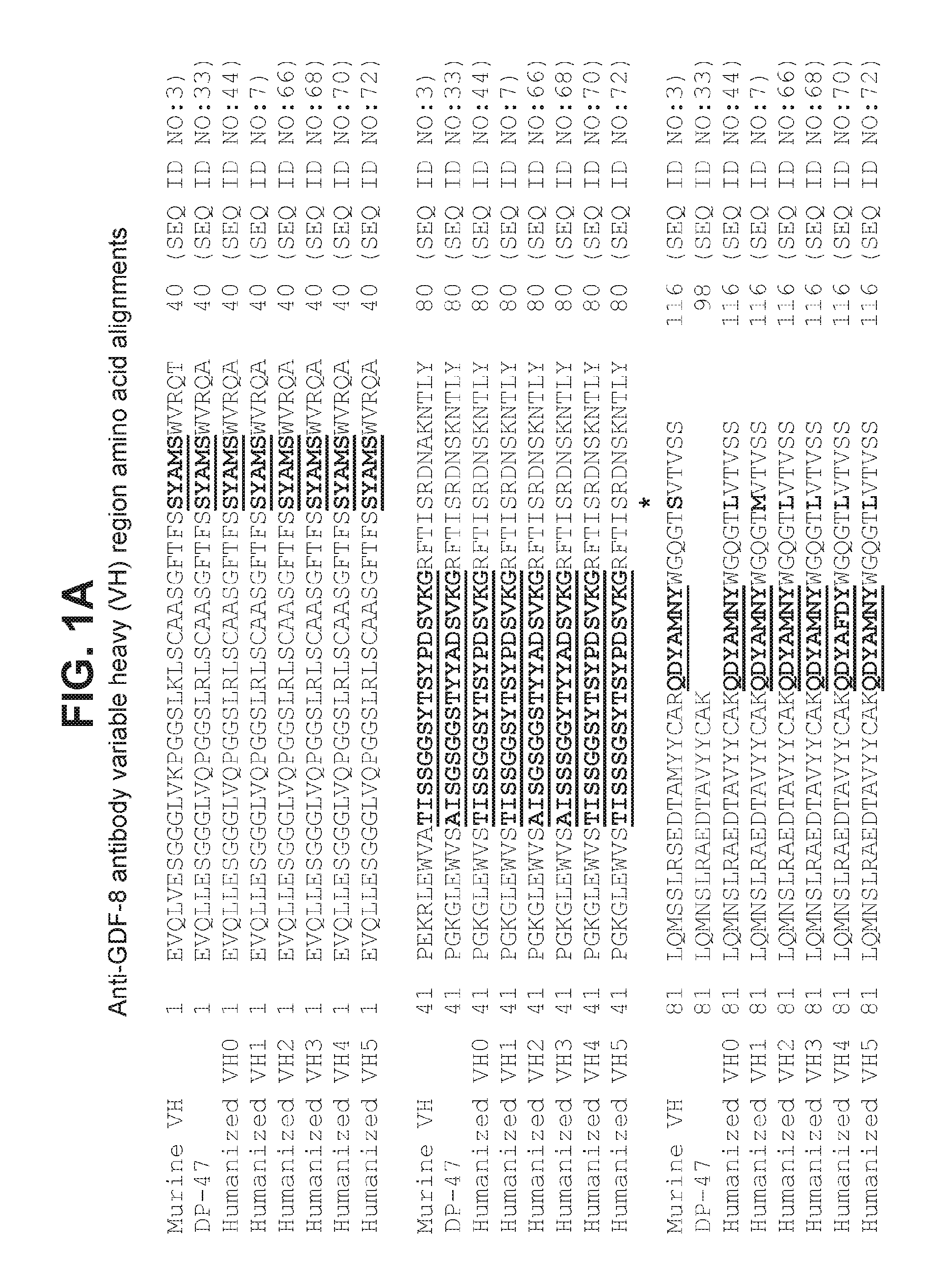
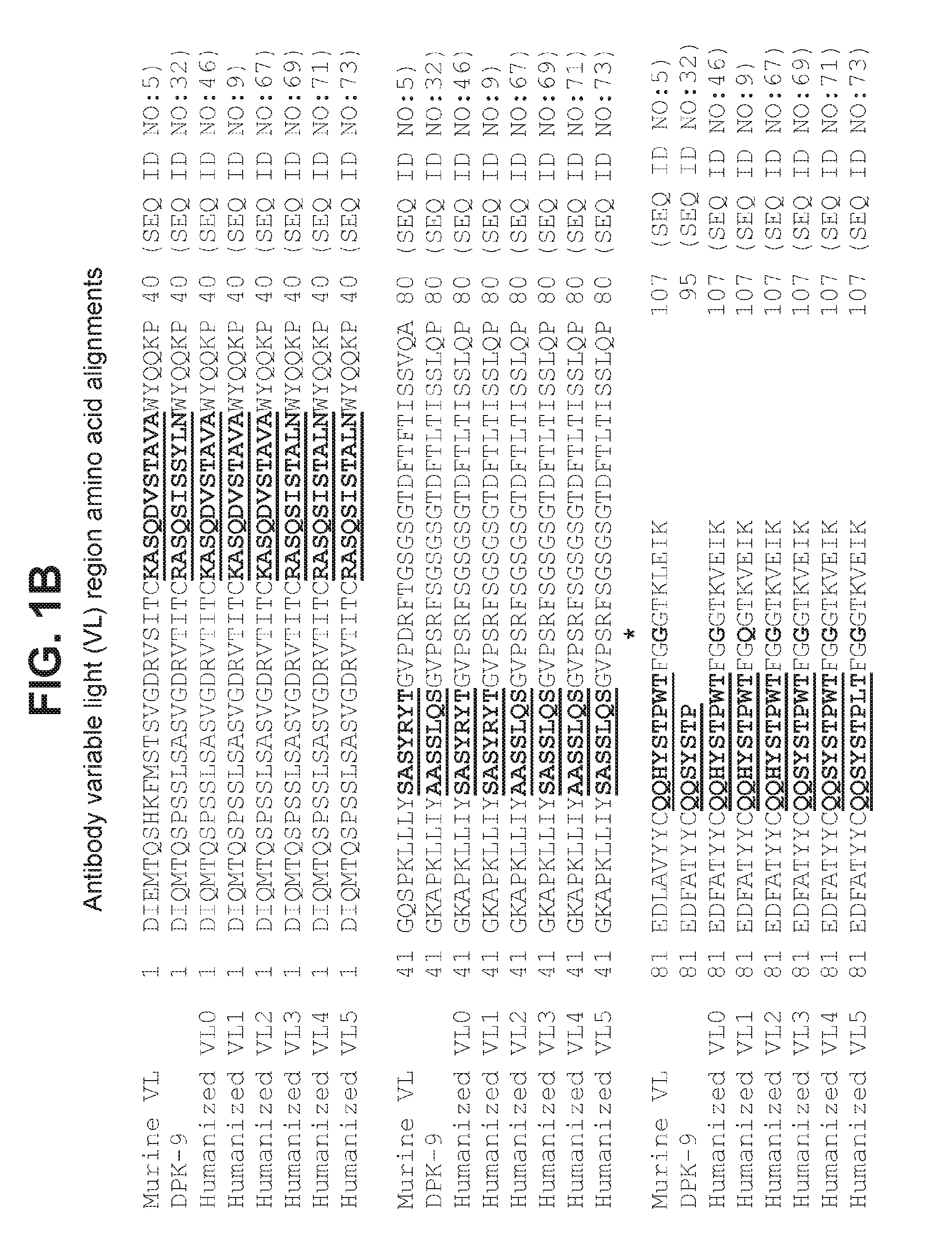
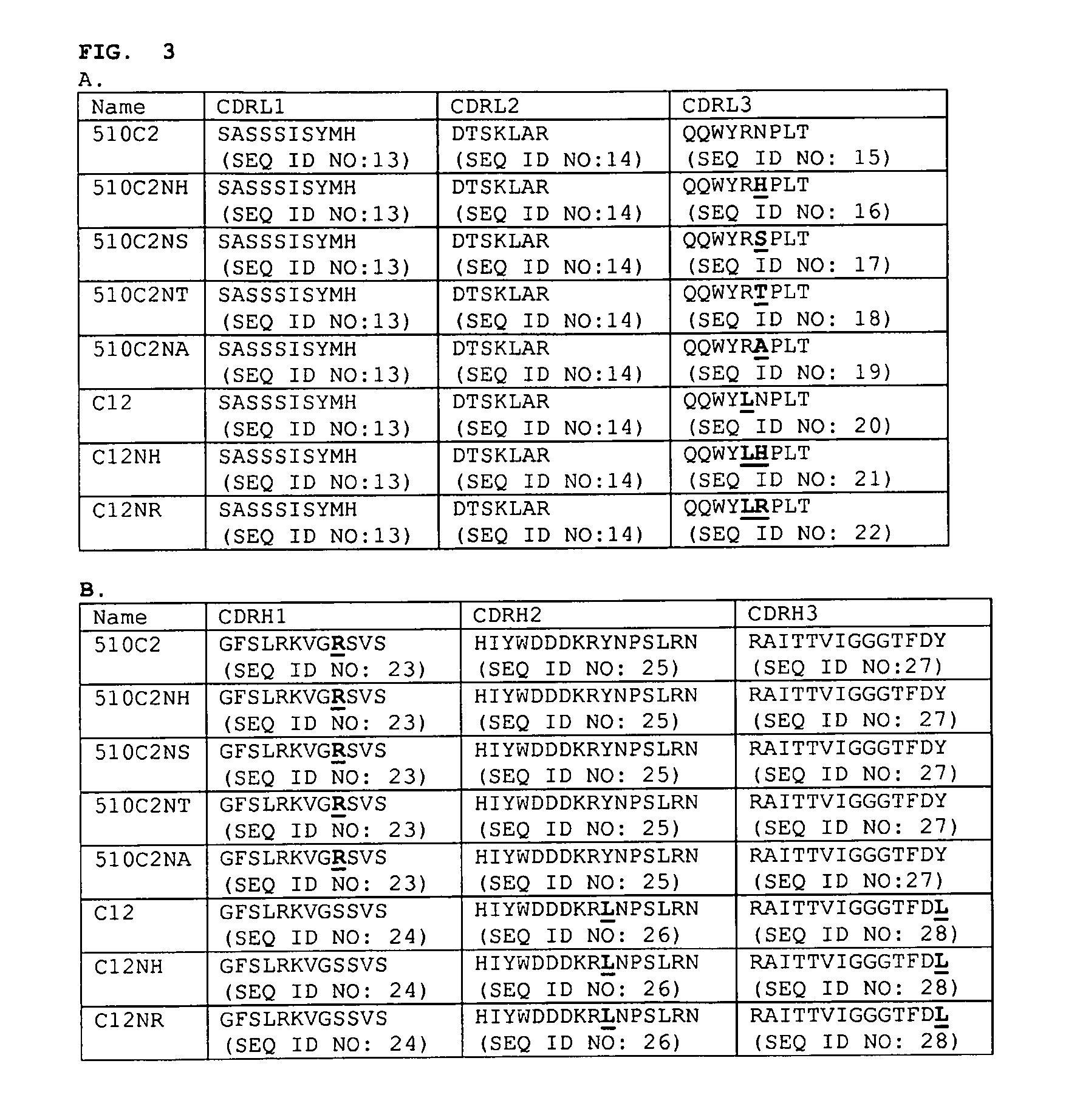
Anti-myostatin antibodies
Anti-myostatin antibodies are identified that are characterized as having high affinity and may be chimeric, humanized or fully human antibodies, immunoconjugates of the antibodies or antigen-binding fragments thereof. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment of various disorders in mammalian and avian species.
Owner:ELI LILLY & CO
Anti-myostatin antibodies
InactiveUS20070178095A1Inhibitory activityQuality improvementAntibacterial agentsMuscular disorderMyostatinDisease
A neutralizing epitope is identified within amino acids 40-64 of the mature form of human myostatin. Antibodies that bind this epitope fall within the scope of the invention and may be murine, chimeric, or humanized antibodies, immunoconjugates of the antibodies or antigen-binding fragments thereof. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment of various disorders in mammals.
Owner:ELI LILLY & CO
Dietary supplement and method of using same
InactiveUS20060204599A1Good for weight lossAppetite suppressantBiocideOrganic active ingredientsDietary supplementPhysical performance
A dietary supplement is provided whereby the daily administration of materials derived from the Acacia plants is provided orally to humans for the purpose of producing or maintaining weight loss as well as improving a person's physical performance and increasing the person's lean muscle mass. The Acacia materials include these portions of the plant that are normally considered waste or inedible, such as leaves, bark, and roots. The materials can be administered in their natural form or as extracts, and can be administered in various ways including capsules and tablets. The Acacia material may also be used as a tea. For weight loss and weight control, the materials may also be administered concurrently with caloric restriction or in the absence of caloric restriction. The materials may also be administered for the purpose of increasing muscle mass concurrently with a high protein diet as well as an exercise program.
Owner:WHEAT JARED R
Regulation of appetite, body weight and athletic function with materials derived from citrus varieties
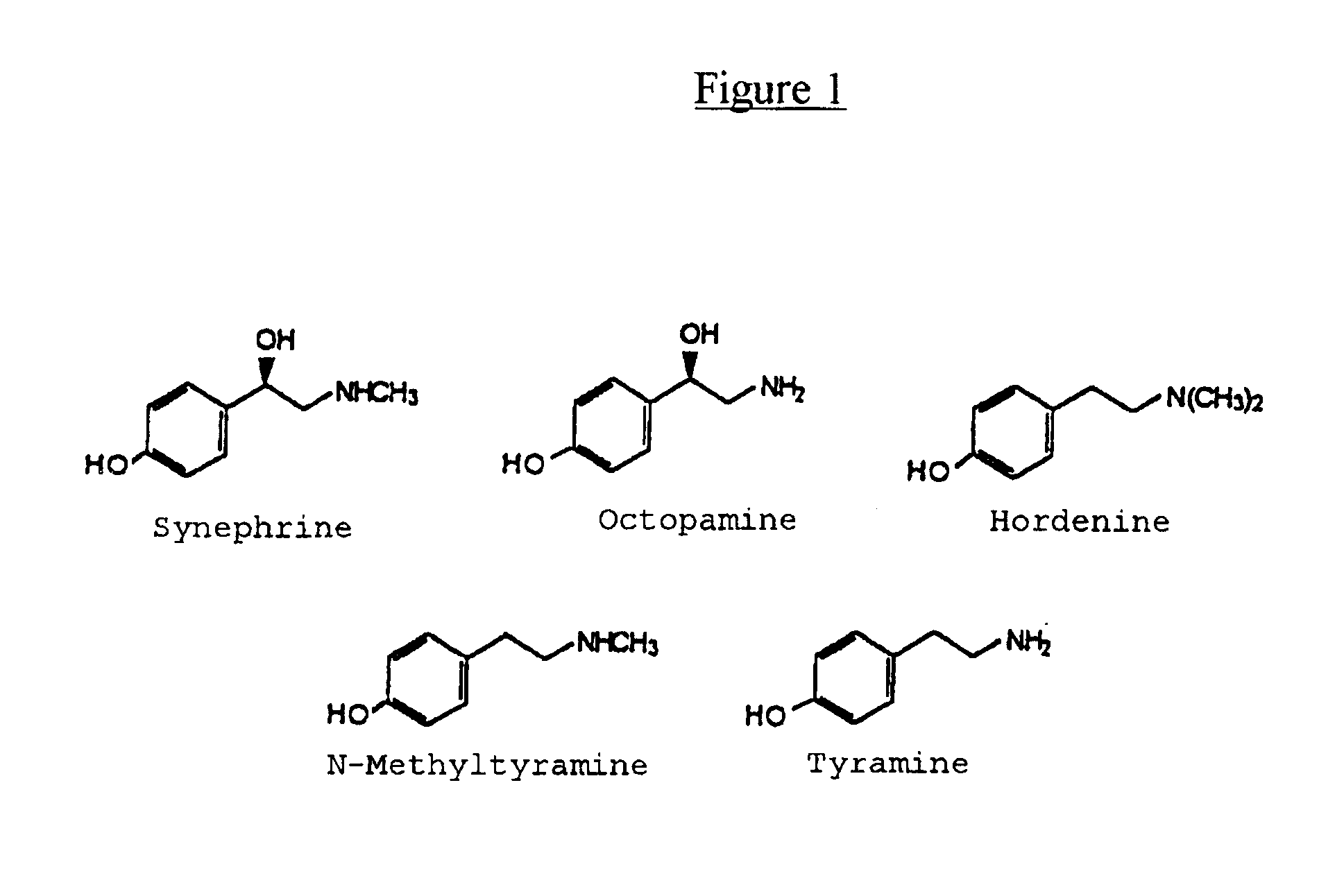
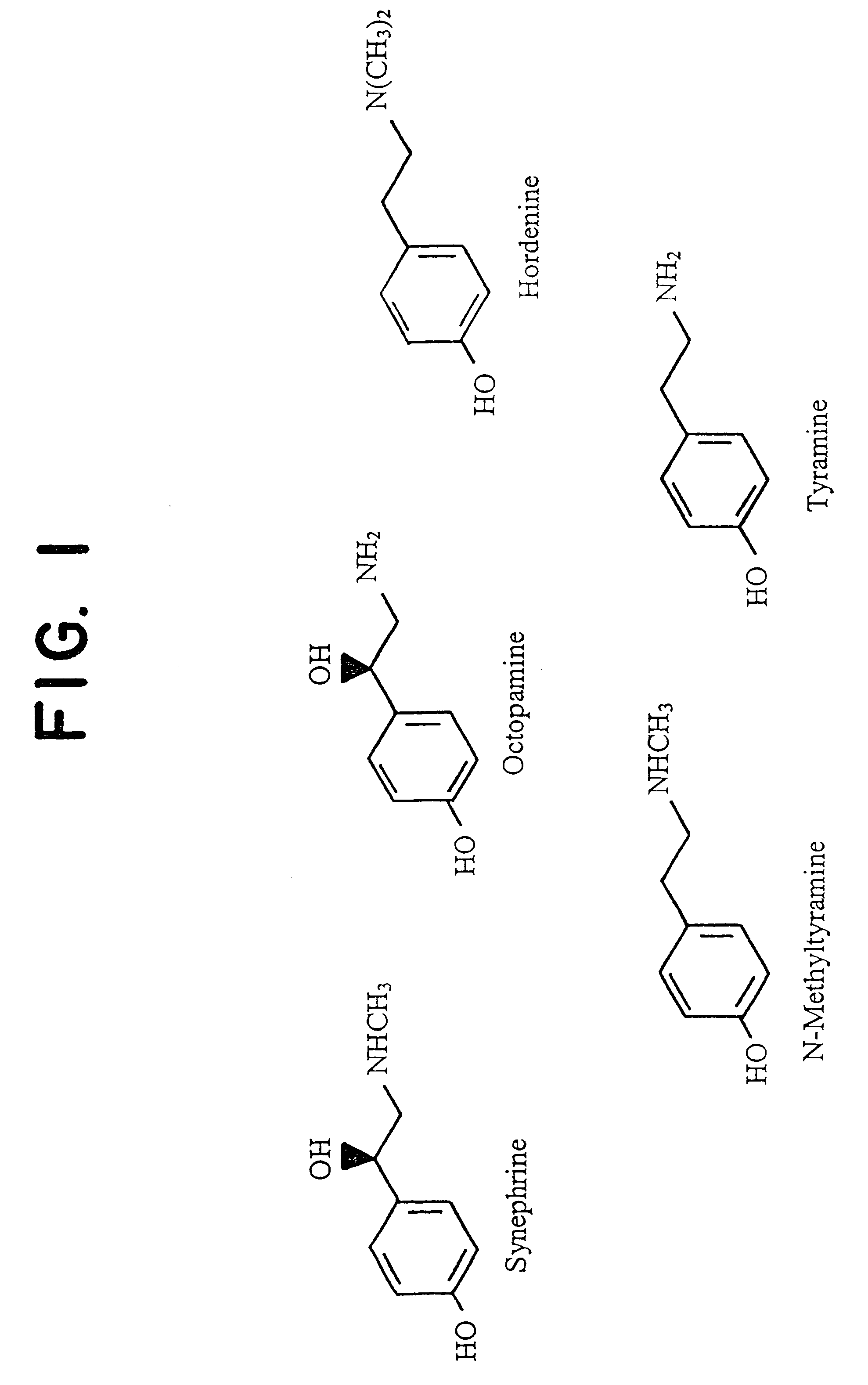
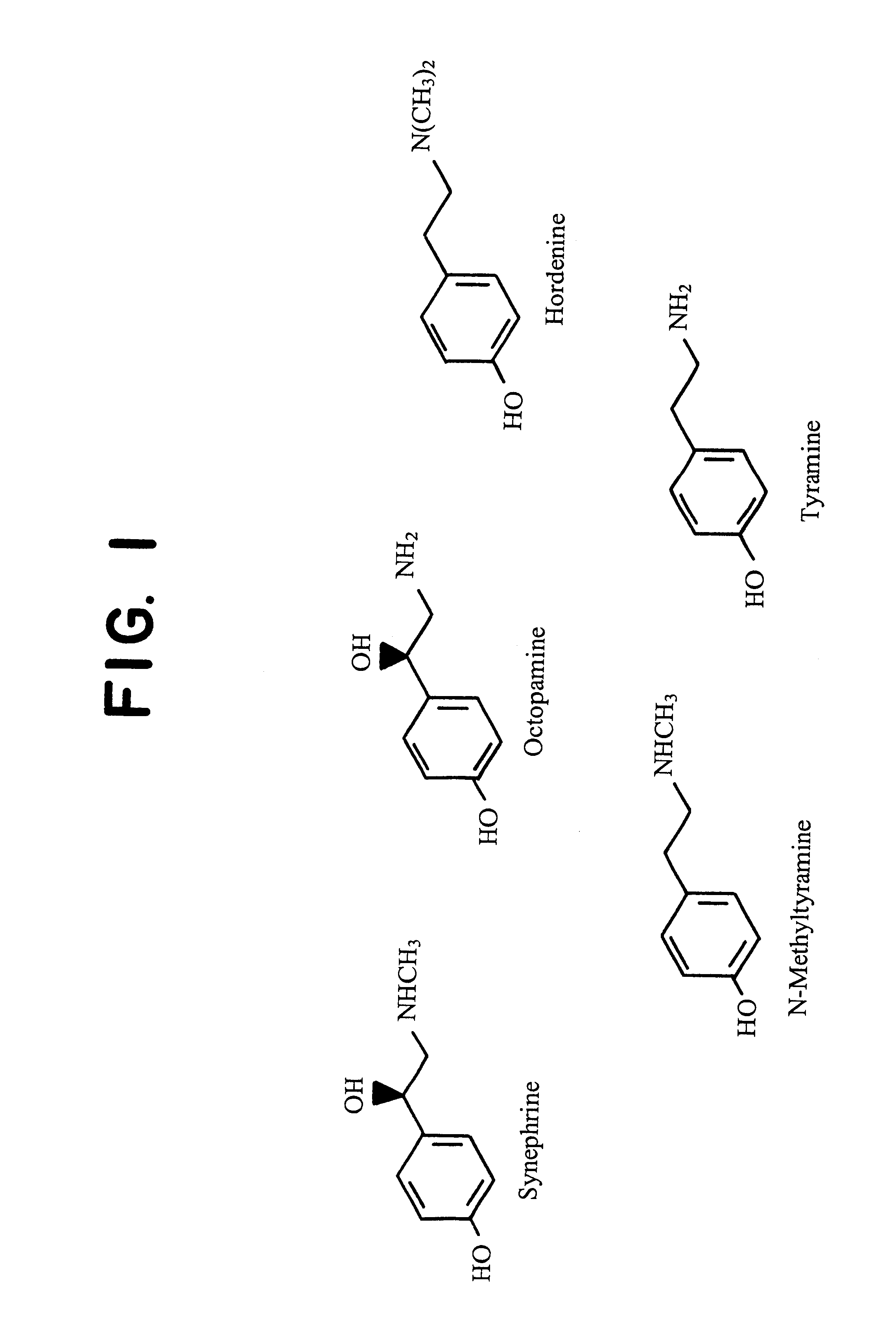
Materials derived from Citrus plants can be administered orally to humans for the purpose of producing or maintaining weight loss as well as for improving the person's physical performance and increasing the person's lean muscle mass. The Citrus materials include those portions of the plant that are normally considered waste or inedible, such as the leaves, peel, and immature, unripe fruit. The materials contain at least one of the alkaloids from the group consisting of synephrine, hordenine, octopamine, tyramine and N-methyltyramine (1). Two species, Citrus aurantium and Citrus reticulata, are particularly useful. The materials can be administered in their natural form or as extracts, and can be administered in various ways including capsules and tablets. The Citrus materials may also be used as a tea. For weight loss and weight control, the materials can be administered concurrently with caloric restriction or in the absence of caloric restriction. The materials may also be administered for the purpose of increasing muscle mass concurrently with a high protein diet as well as with an exercise program.
Owner:STEVENS INSTITUTE OF TECHNOLOGY +1
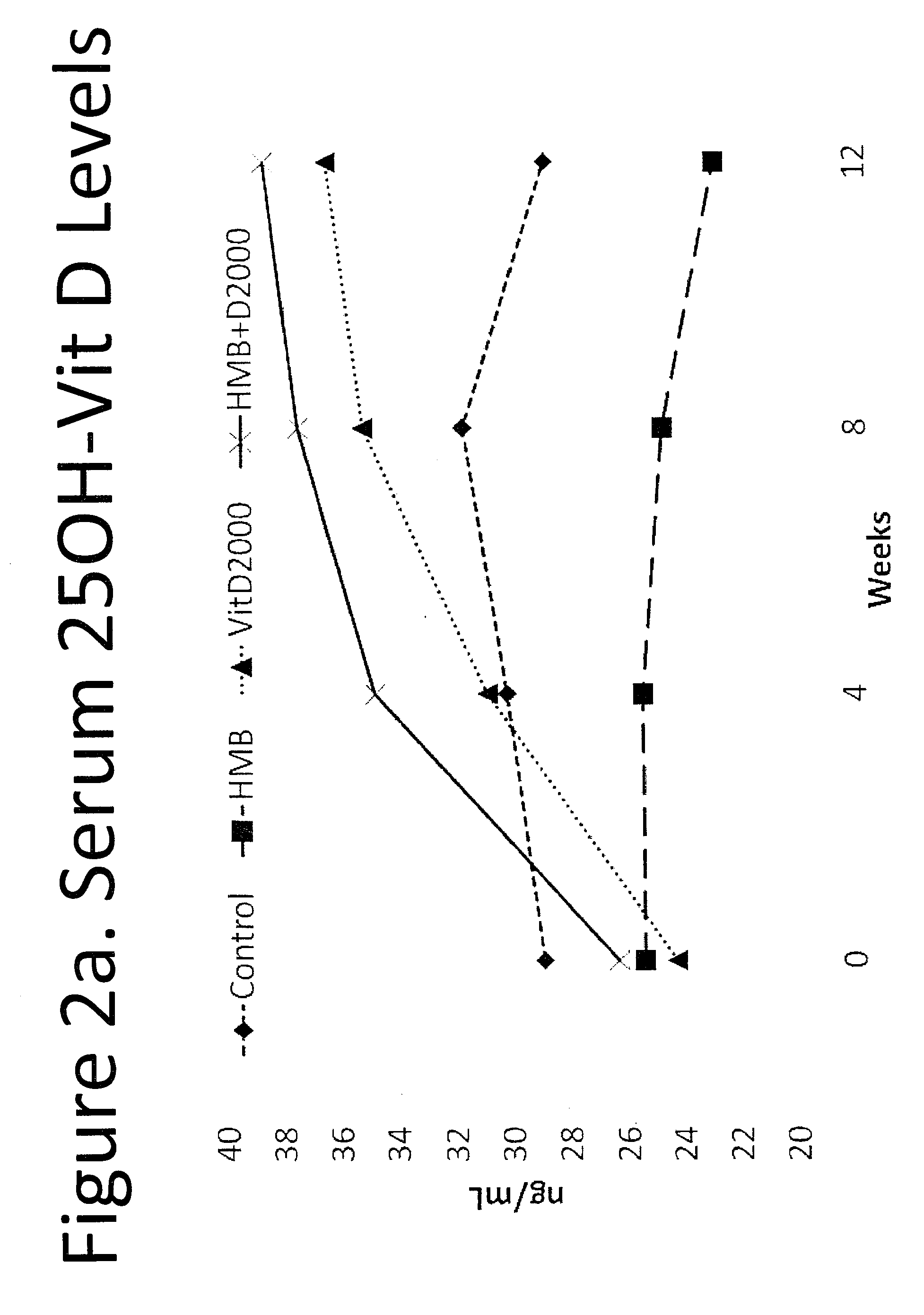
Nutritional Intervention for Improving Muscular Function and Strength
ActiveUS20100179112A1Improve muscle massHigh strengthBiocideVitamin food ingredientsMuscle functionsUltimate tensile strength
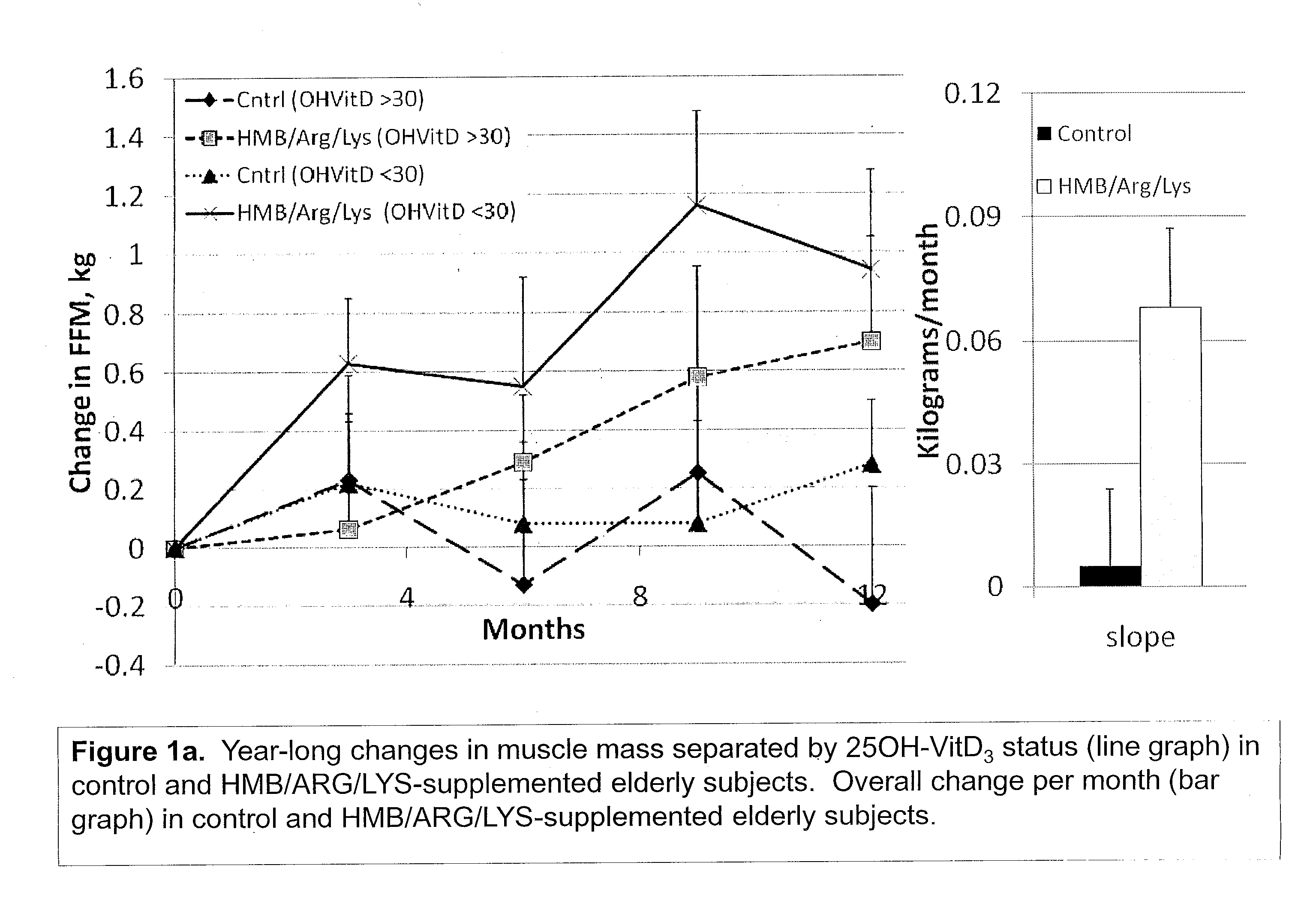
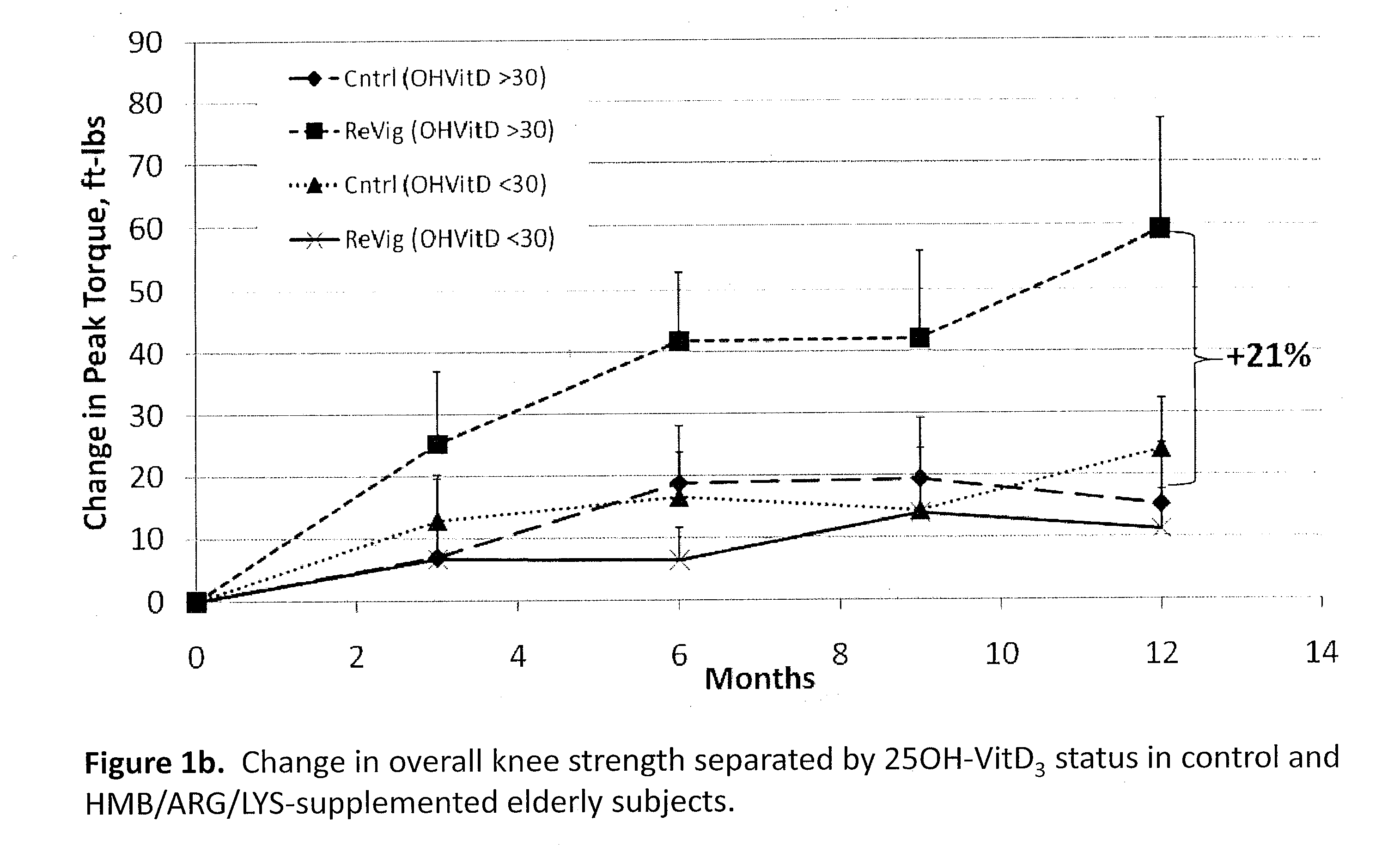
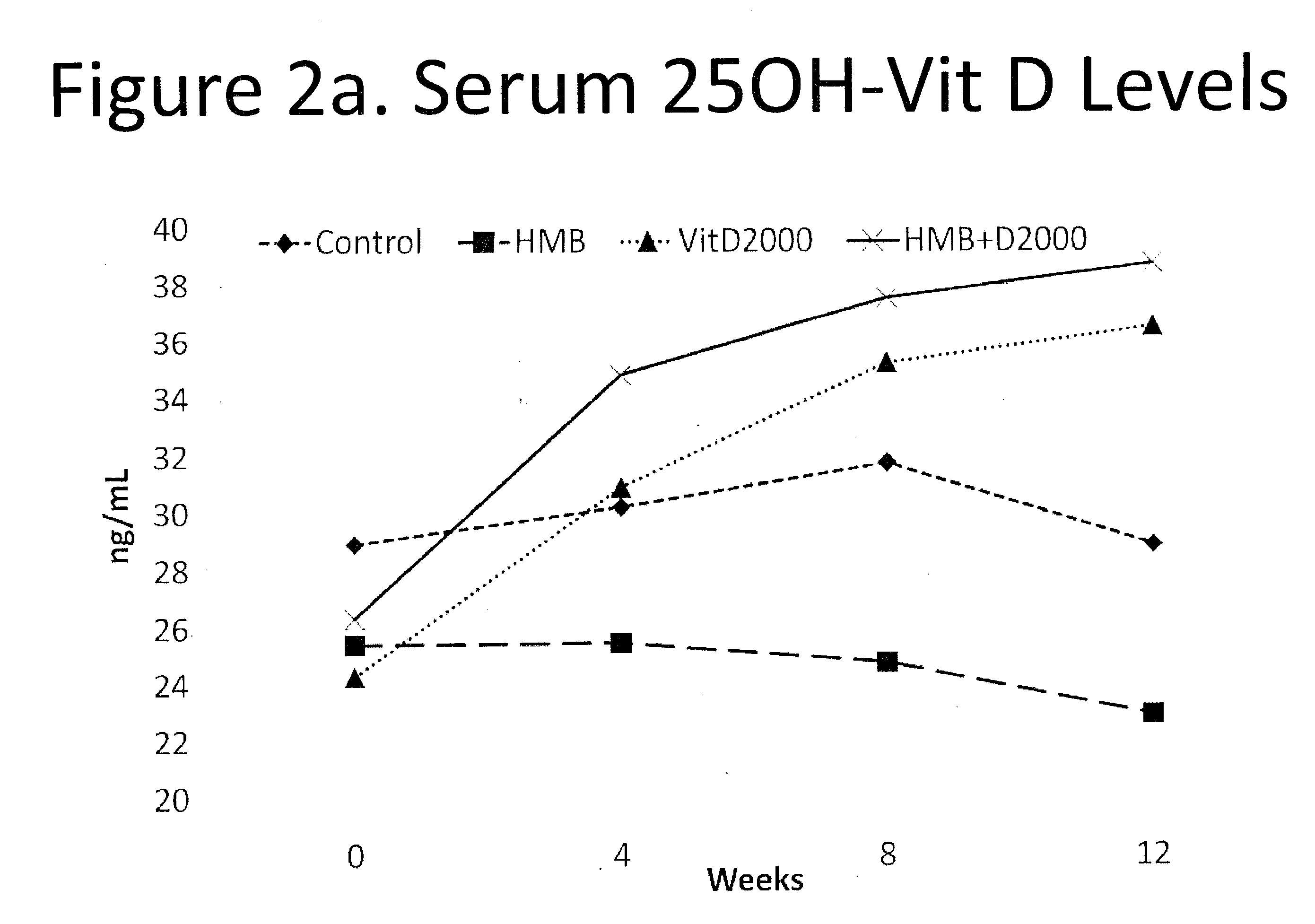
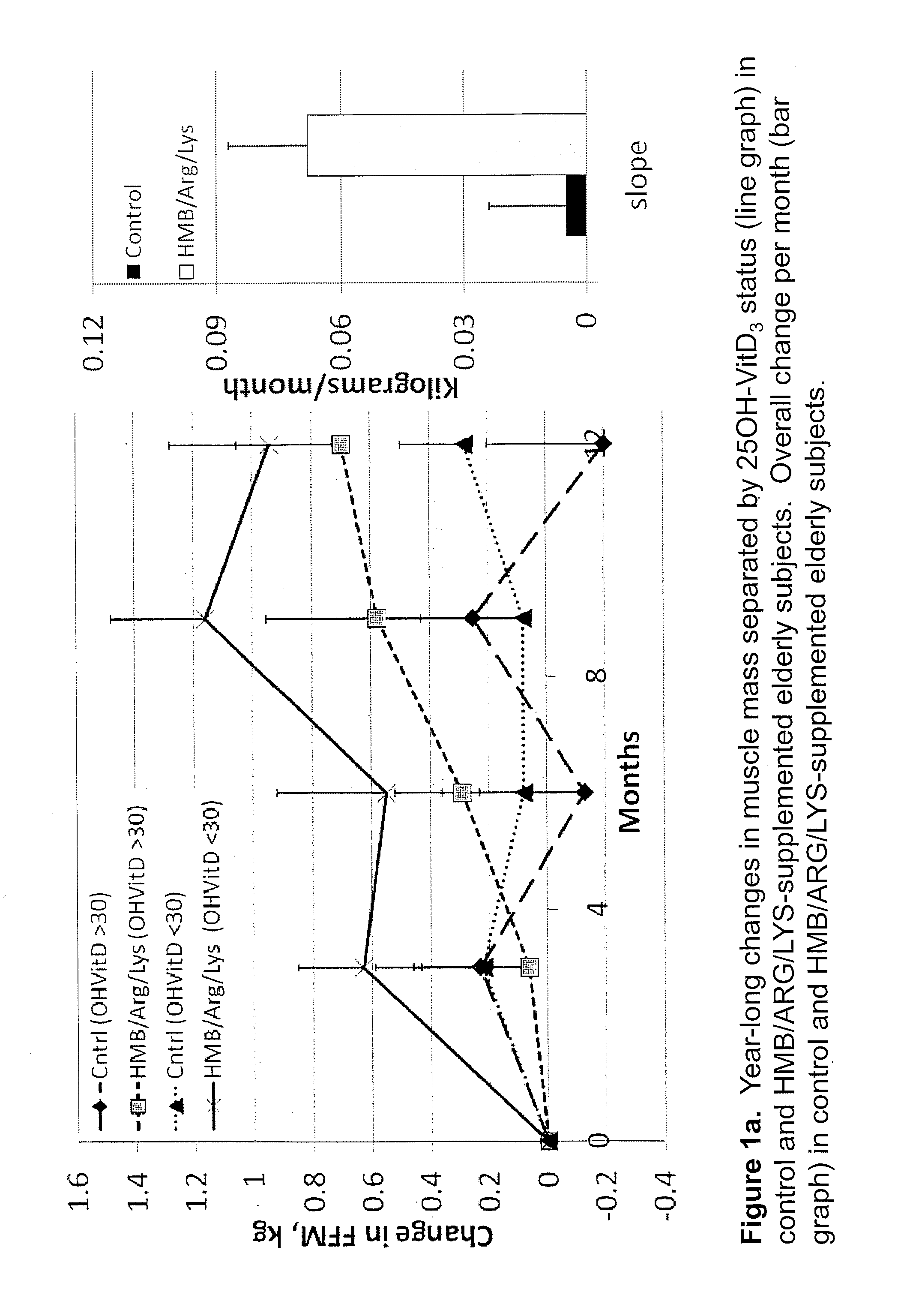
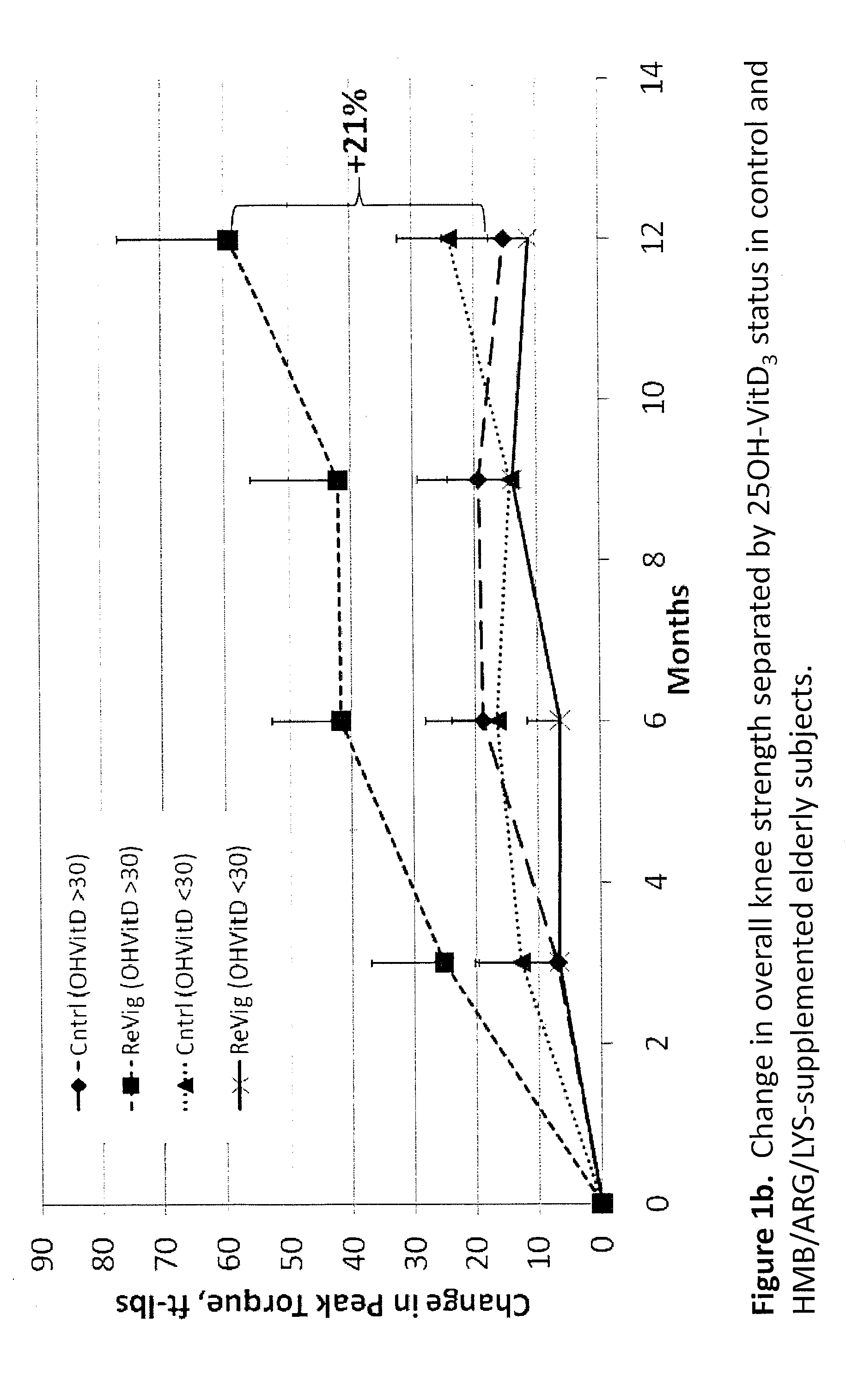
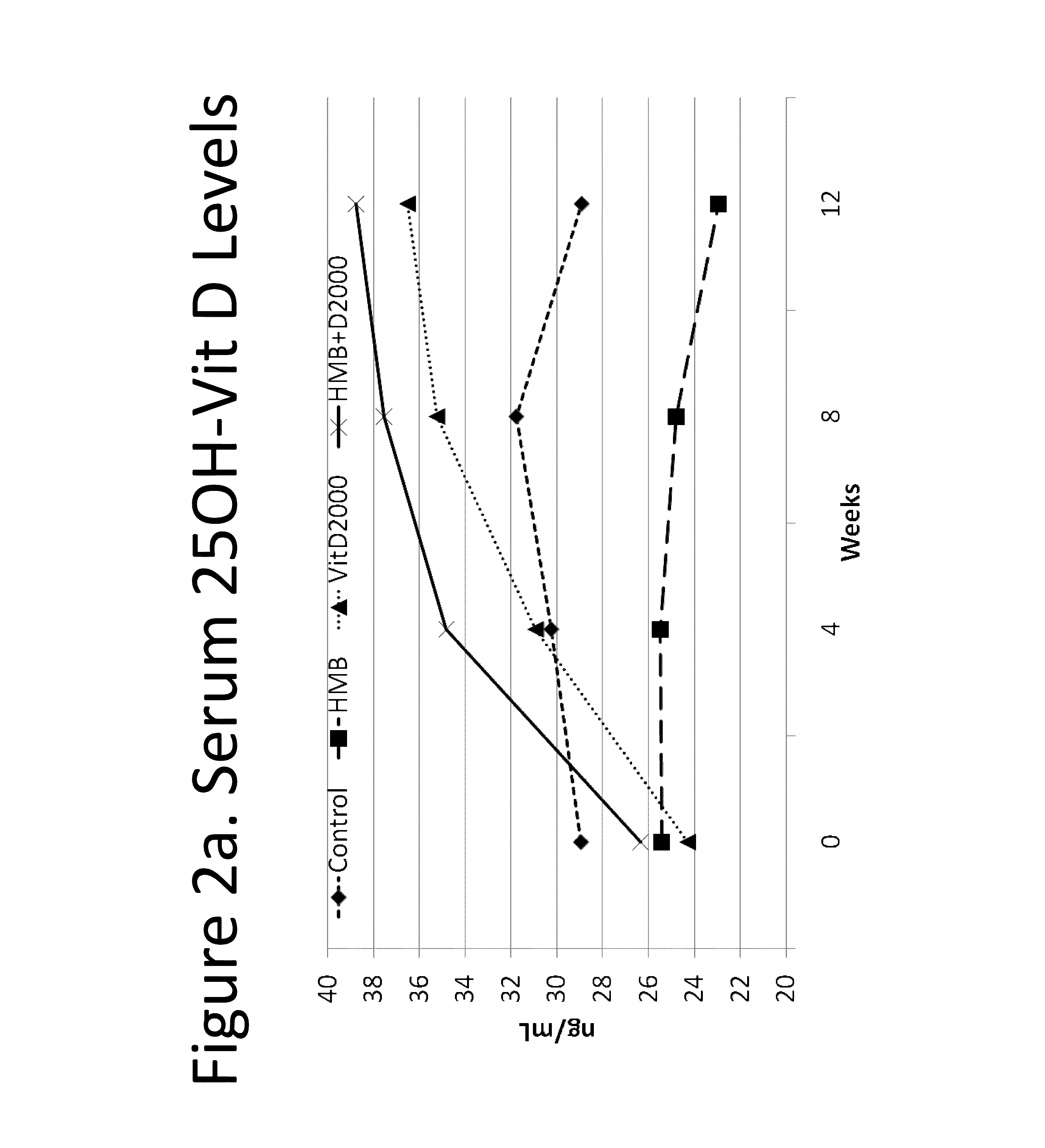
The present invention provides a composition comprising HMB and Vitamin D. Methods of administering HMB and Vitamin D to an animal are also described. Vitamin D and HMB are administered to increase muscle mass, strength, and functionality. The combination of Vitamin D and HMB together has a synergistic effect, which results in a surprising and unexpected level of improvement in muscle mass, strength and functionality.
Owner:METABOLIC TECH
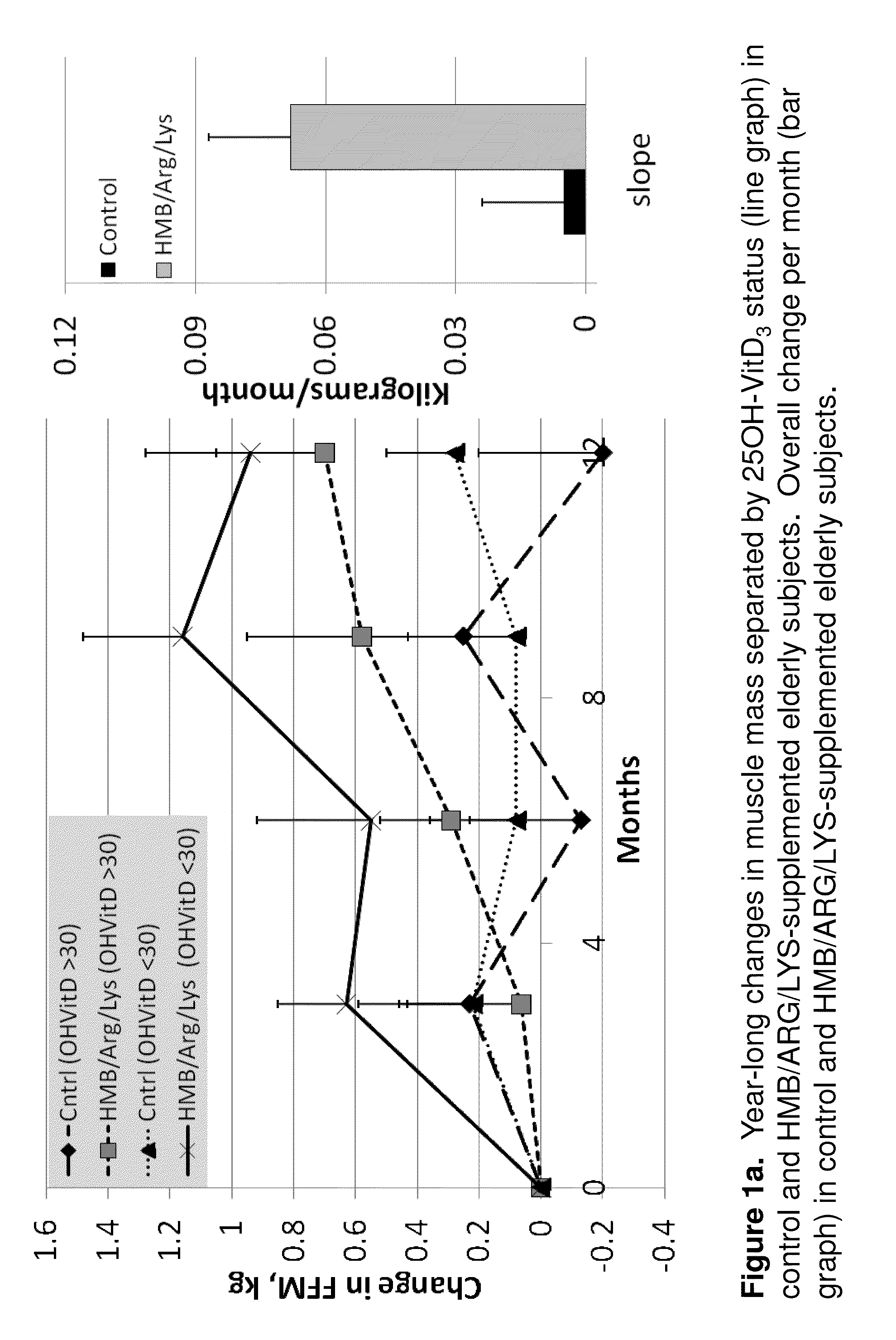
Compositions and methods for increasing muscle mass, strength, and functional performance in the elderly
InactiveUS7790688B2Add additional massImprove performanceBiocideMetabolism disorderUltimate tensile strengthBiology
Owner:ENERGY LIGHT
Anti-myostatin antibodies
A neutralizing epitope is identified within amino acids 40-64 of the mature form of human myostatin. Antibodies that bind this epitope with high affinity preferentially bind GDF-8 over GDF-11 and may be chimeric, humanized or fully human antibodies, immunoconjugates of the antibodies or antigen-binding fragments thereof. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment of various disorders in mammalian and avian species.
Owner:ELI LILLY & CO
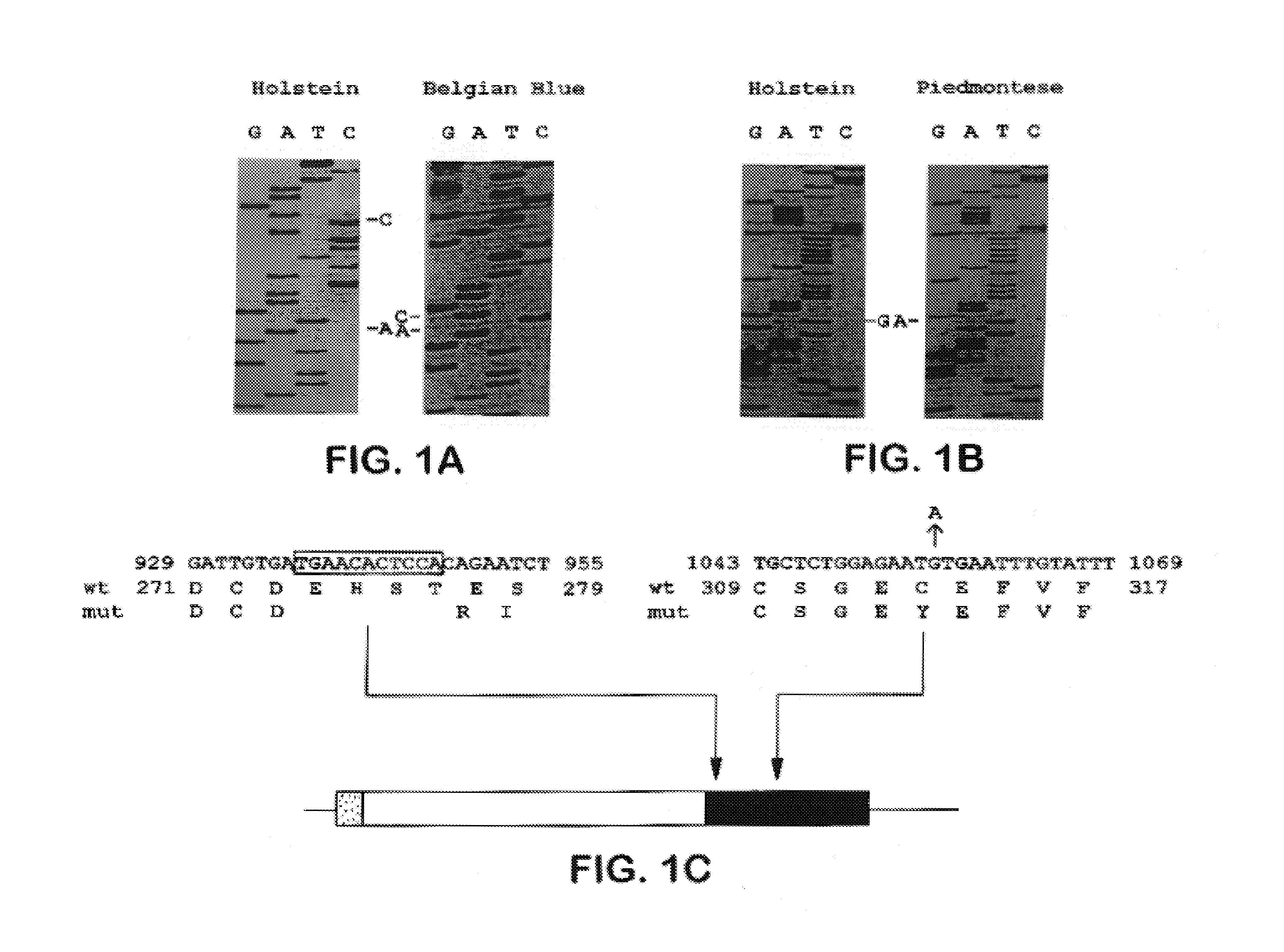
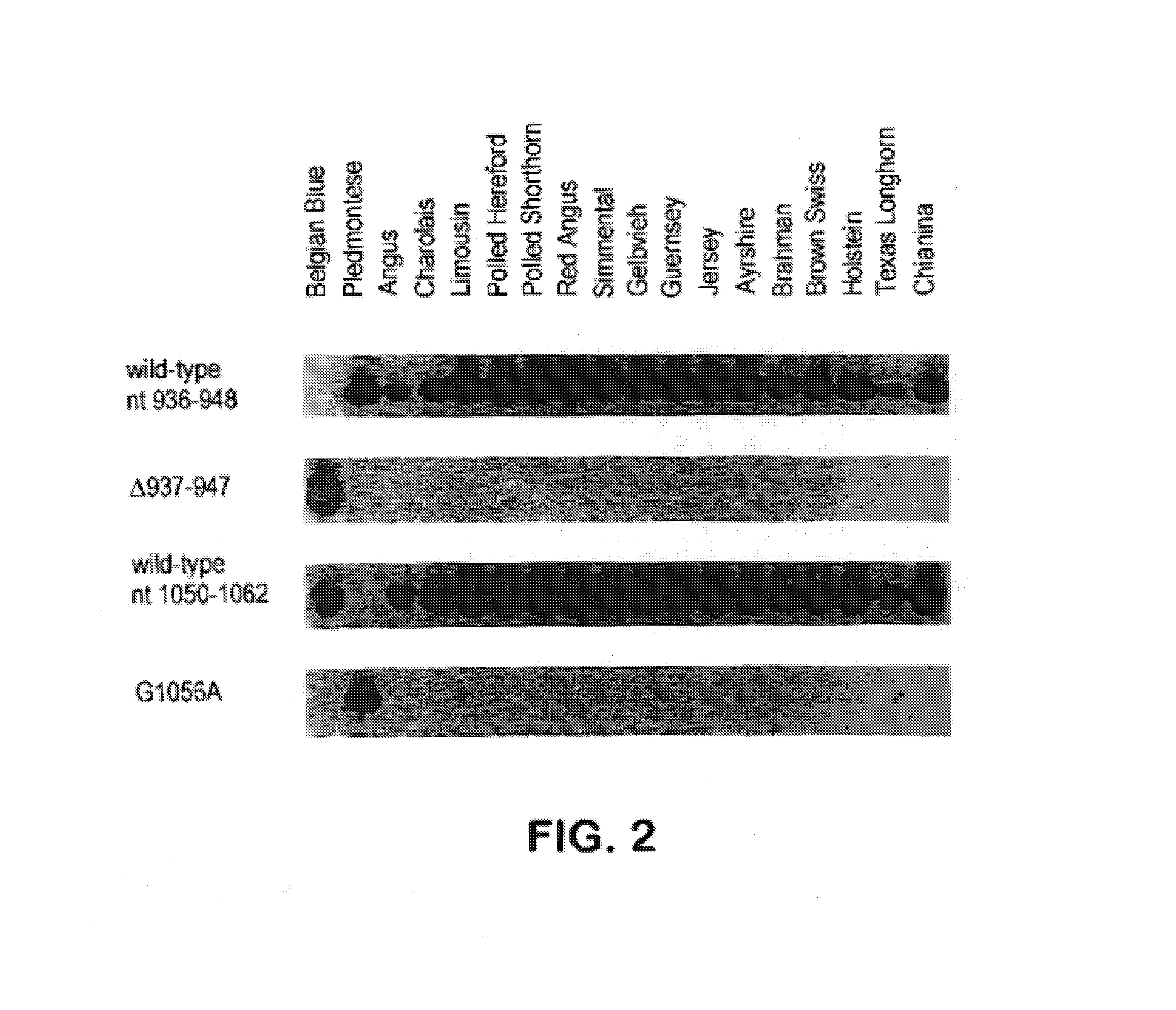
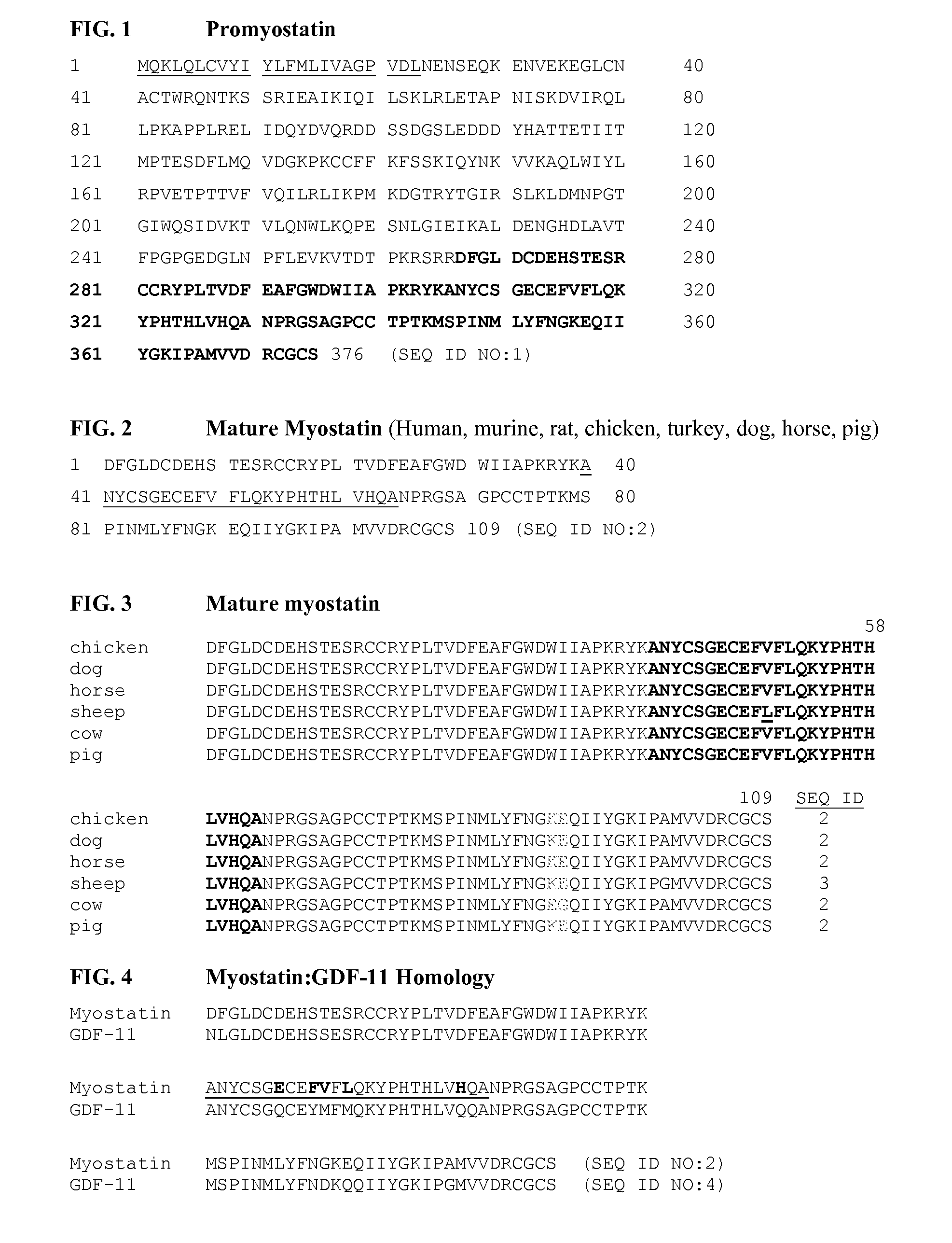
Methods for detection of mutations in myostatin variants
Methods for detecting allelic variants of the myostatin (growth and differentiation factor-8) gene are provided. Specifically provided are methods of identifying subjects having or having a predisposition for increased muscle mass as compared to subjects having wild-type myostatin. Increased muscle mass is particularly desirable for identification of animals used to produce food products, including bovine, porcine, ovine, avian and piscine species.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Antagonist antibodies against GDF-8 and uses therefor
The disclosure provides improved neutralizing anti-GDF-8 antibodies capable of substantially higher levels of expression in host cells compared to previous anti-GDF-8 antibodies. Also provided are methods of using compositions comprising such antibodies to increase muscle mass or strength, and to treat or prevent muscular disorders, neuromuscular disorders, metabolic disorders, adipose tissue disorders or bone disorders.
Owner:PFIZER INC
Antagonist antibodies against gdf-8 and uses therefor
InactiveUS20130336982A1Improve performanceLoss of massSugar derivativesMetabolism disorderDiseaseNeuromuscular disease
The disclosure provides improved neutralizing anti-GDF-8 antibodies capable of substantially higher levels of expression in host cells compared to previous anti-GDF-8 antibodies. Also provided are methods of using compositions comprising such antibodies to increase muscle mass or strength, and to treat or prevent muscular disorders, neuromuscular disorders, metabolic disorders, adipose tissue disorders or bone disorders.
Owner:PFIZER INC
Botanically Derived Composition and a Process Thereof
ActiveUS20090281057A1Maintain healthy blood glucose levelAvoid delayOrganic active ingredientsBiocideDiabetes mellitusMedicine
The present invention relates to a novel botanical compound as provided in structural formula I optionally along with excipients for improving body composition and the factors related to the pre-diabetic and diabetic conditions. It also relates to a process of manufacture of the novel botanical compound for improving body composition and factors related to the pre-diabetic and diabetic conditions. The present invention also relates to the use of the novel botanical compound for improving body composition, reducing body fat, increasing muscle mass, enhancing strength and improving impaired glucose metabolism. The present invention also relates to the use of the novel botanical compound for the improvement of factors related to the pre-diabetic and diabetic conditions.
Owner:INDUS BIOTECH PRICATE
Methods of increasing muscle mass or muscle strength using antibody inhibitors of GDF-8
InactiveUS7731961B1Reduction in one or more of the biological activitiesReduced activityNervous disorderPeptide/protein ingredientsMuscle strengthAntibody inhibitor
The disclosure provides novel antibodies against growth and differentiation factor-8 (GDF-8), including antibody fragments, which inhibit GDF-8 activity in vitro and in vivo. The disclosure also provides methods for diagnosing, preventing, or treating degenerative disorders of muscle, bone, or insulin metabolism.
Owner:WYETH LLC
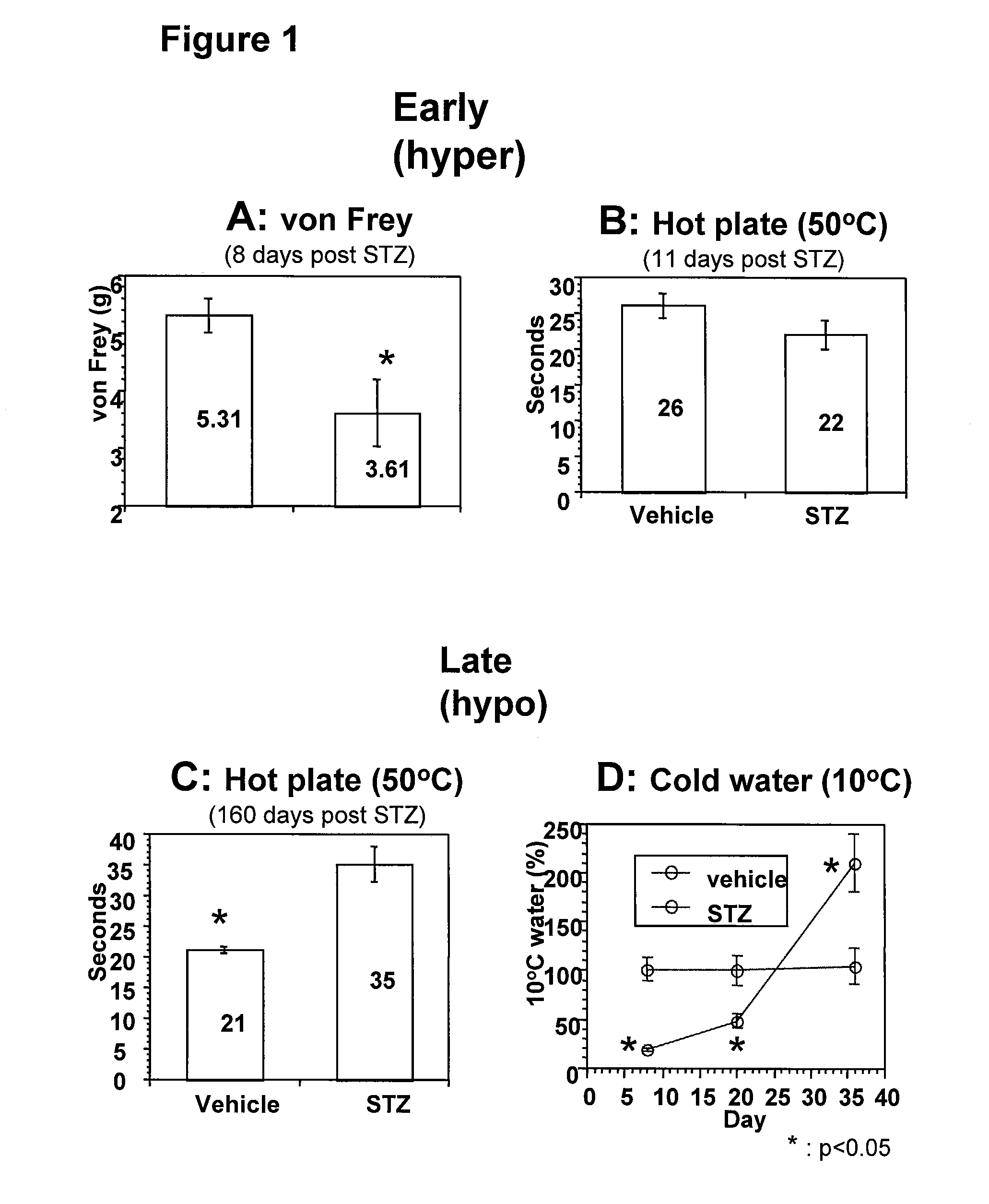
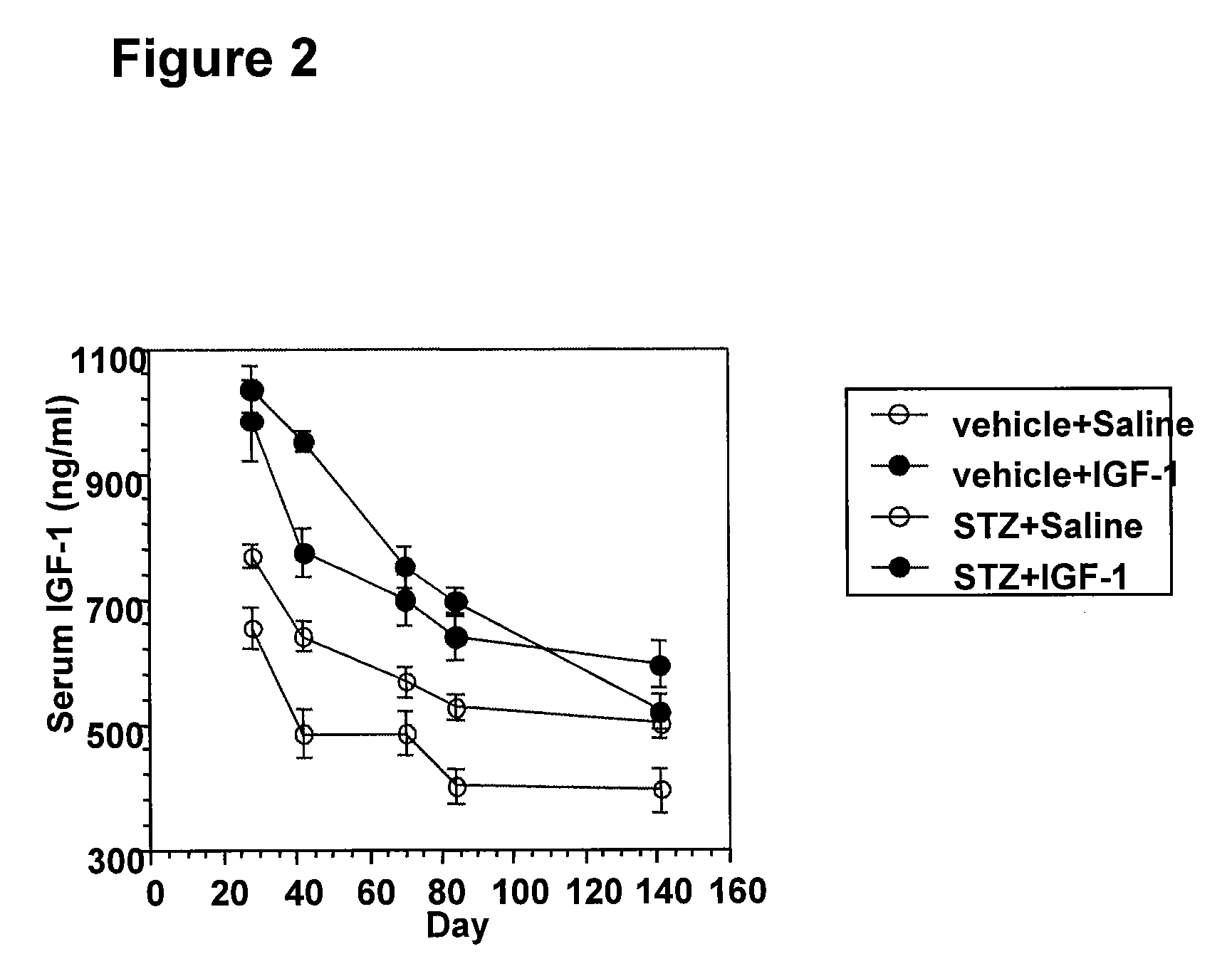
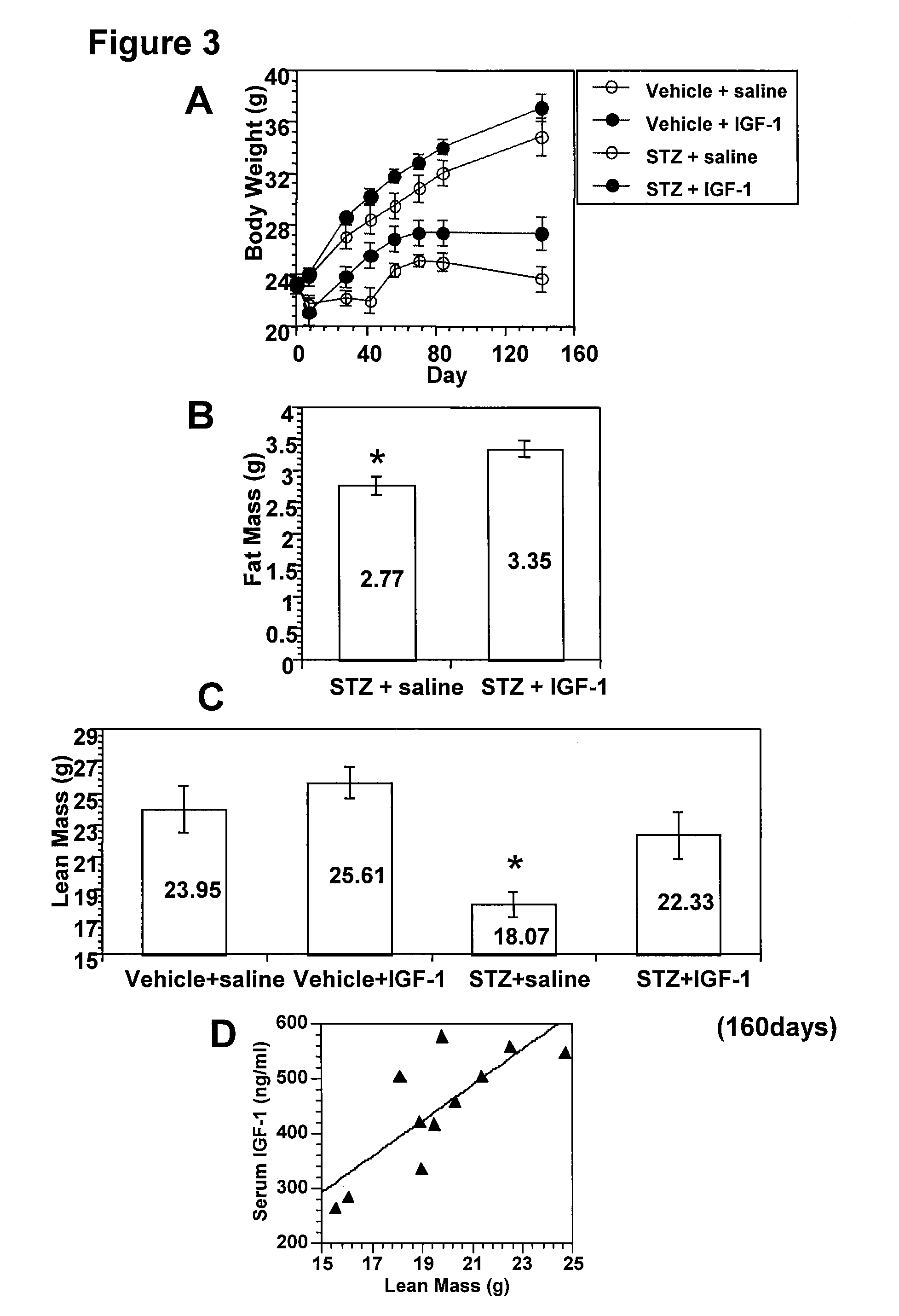
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
Method for increasing muscle mass and strength
ActiveUS20120141448A1Good effectRapid and intenseBiocideHeavy metal active ingredientsHerbal supplementUltimate tensile strength
Phosphatidic acid is administered orally to increase muscle mass and strength in exercising mammals. Phosphatidic acid is administered orally to aging, bedridden or cachectic patients to improve nitrogen balance. The preferred form of phosphatidic acid for administration is phosphatidic acid-enriched lecithin. Creatine is co-administered orally to increase the muscle-building and strength effect. Other suggested additives include nutritional and herbal supplements, micronutrients and hormones.
Owner:CHEMI NUTRA
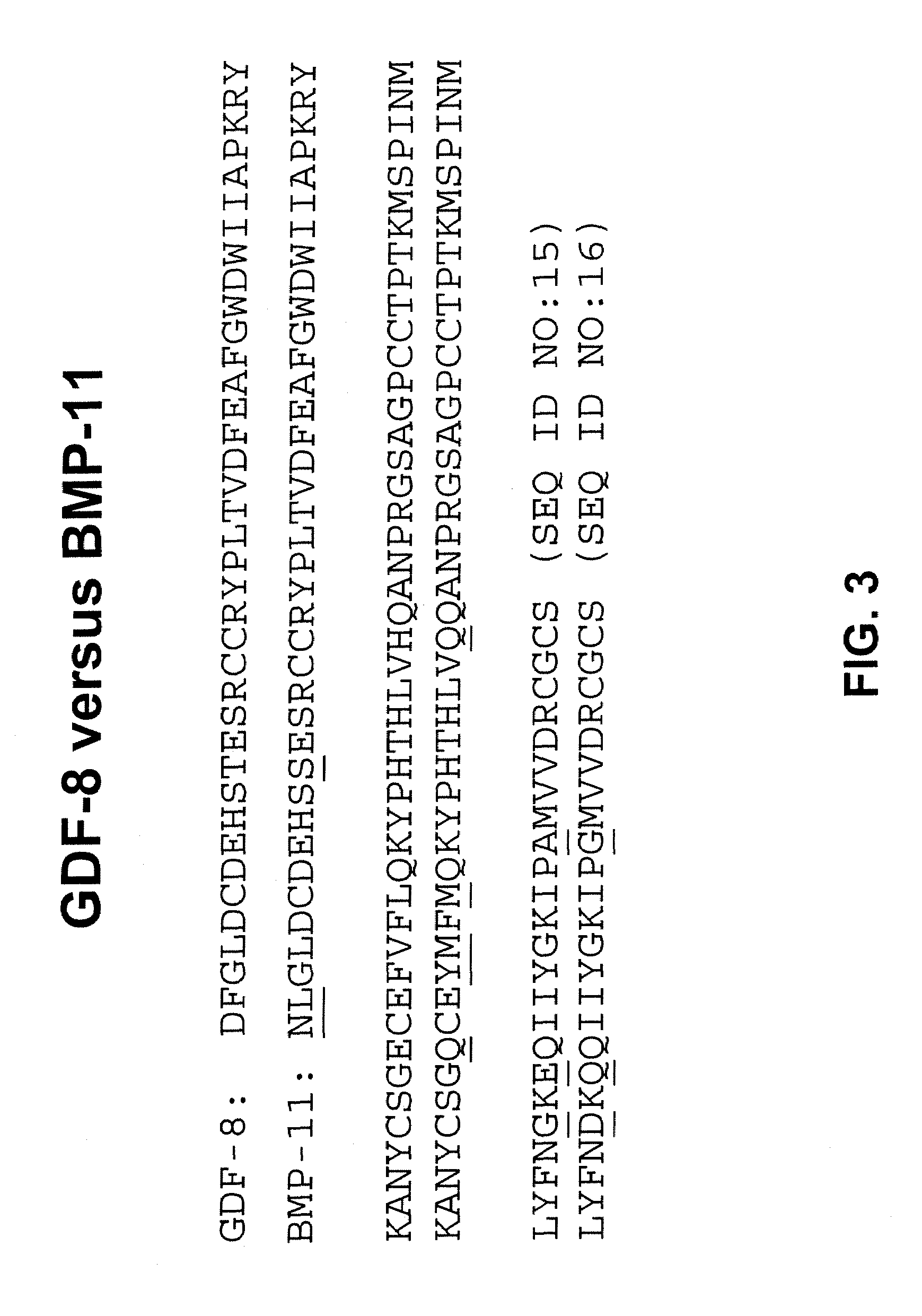
Method for down-regulating GDF-8 activity using immunogenic GDF-8 analogues
InactiveUS7070784B1Small sizeQuality improvementAntibody mimetics/scaffoldsGenetic material ingredientsMyostatinVaccination
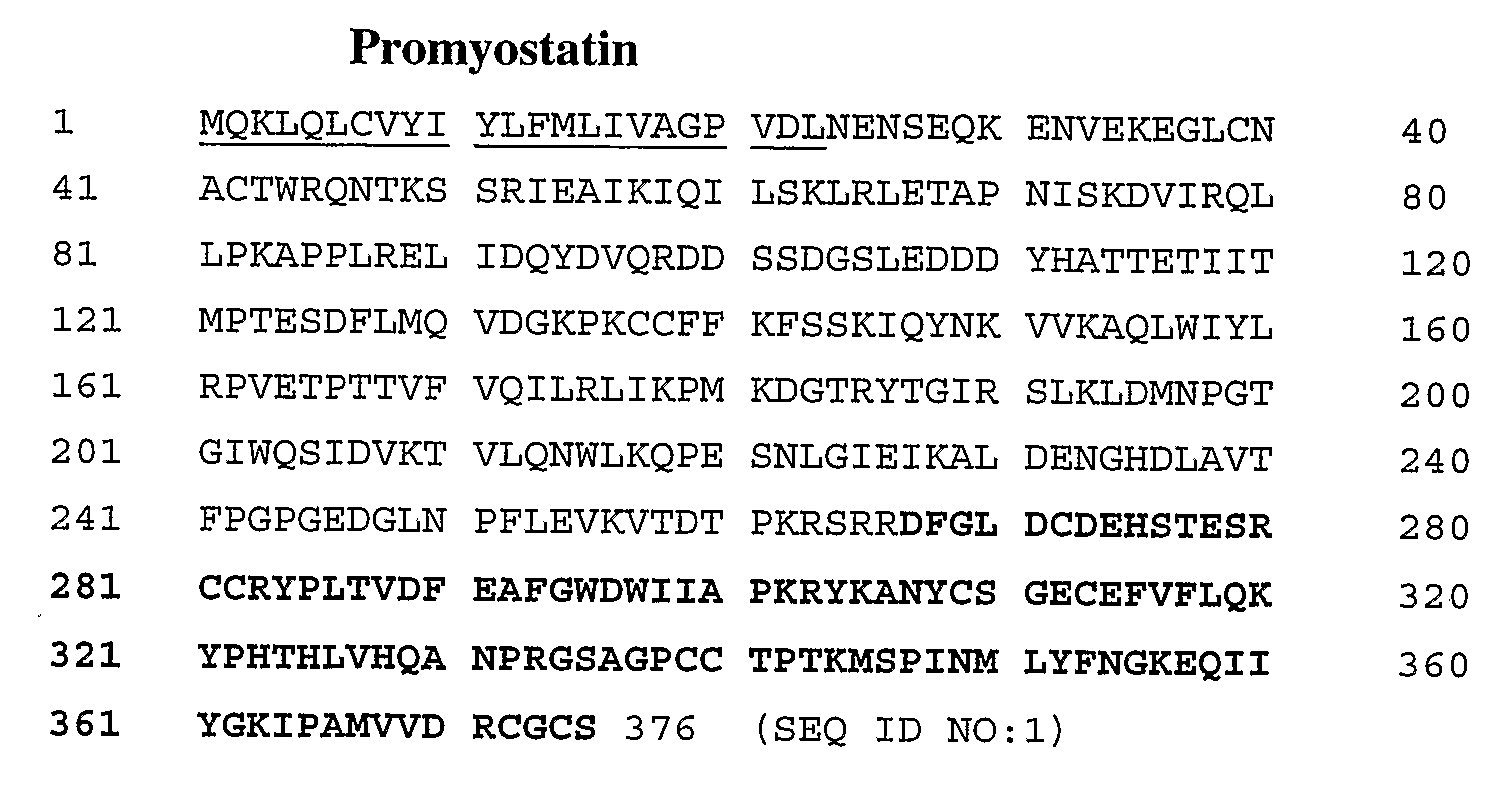
Disclosed are novel methods for increasing muscle mass by means of immunization against Growth Differentiation Factor 8 (GDF-8, myostatin). Immunization is preferably effected by administration of analogues of GDF-8 which are capable of inducing antibody production against homologous GDF-8. Especially preferred as an immunogen is homologous GDF-8 which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes while substantially preserving the tertiary structure of the homologous GDF-8. Also disclosed are nucleic acid vaccination against GDF-8 and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for identification of useful immunogenic GDF-8 analogues, methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:PHARMEXA
Methods for inducing weight loss in a human with materials derived from Citrus varieties
Materials derived from Citrus plants can be administered orally to humans for the purpose of producing or maintaining weight loss as well as improving the person's physical performance and increasing the person's lean muscle mass. The Citrus materials include those portions of the plant that are normally considered waster or inedible, such as the leaves, peel and immature, unripe fruit. The materials contain as least one of the alkaloids from the group consisting of synephrine, hordenine, octopamine, tyramine and N-methylamine. Two species, Citrus aurantium and Citrus reticulata, are particularly useful. The materials can be administered in their natural form or as extracts, and can be administered in various ways including capsules and tablets. The Citrus materials may also be used as a tea. For weight loss and weight control, the materials can be administered concurrently with caloric restriction or in the absence of caloric restriction. The materials may also be administered for the purpose of increasing muscle mass concurrently with a high protein diet as well as with an exercise program.
Owner:ADVANTRA Z INC
Nutritional intervention for improving muscular function and strength
ActiveUS8815280B2Increasing muscle mass and strength and functionalityImprove functionalityBiocideVitamin food ingredientsMuscle functionsUltimate tensile strength
The present invention provides a composition comprising HMB and Vitamin D. Methods of administering HMB and Vitamin D to an animal are also described. Vitamin D and HMB are administered to increase muscle mass, strength, and functionality. The combination of Vitamin D and HMB together has a synergistic effect, which results in a surprising and unexpected level of improvement in muscle mass, strength and functionality.
Owner:METABOLIC TECH
Anti-myostatin antibodies
Anti-myostatin antibodies are identified that are characterized as having high affinity and may be chimeric, humanized or fully human antibodies, immunoconjugates of the antibodies or antigen-binding fragments thereof. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment of various disorders in mammalian and avian species.
Owner:ELI LILLY & CO
Anti-myostatin antibodies
InactiveUS8063188B2Immunoglobulins against cytokines/lymphokines/interferonsMuscular disorderMyostatinInhibin hormone
Monoclonal anti-myostatin antibodies that preferentially bind myostatin over GDF-11, have strong binding affinity to myostatin and are resistant to chemical degradation. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment or prevention of various disorders in mammalian and avian species.
Owner:ELI LILLY & CO
Methods for inducing weight loss in a human with materials derived from citrus varieties
Materials derived from Citrus plants can be administered orally to humans for the purpose of producing or maintaining weight loss as well as for improving the person's physical performance and increasing the person's lean muscle mass. The Citrus materials include those portions of the plant that are normally considered waste or inedible, such as the leaves, peel, and immature, unripe fruit. The materials contain at least one of the alkaloids from the group consisting of synephrine, hordenine, octopamine, tyramine and N-methyltyramine (1). Two species, Citrus aurantium and Citrus reticulata, are particularly useful. The materials can be administered in their natural form or as extracts, and can be administered in various ways including capsules and tablets. The Citrus materials may also be used as a tea. For weight loss and weight control, the materials can be administered concurrently with caloric restriction or in the absence of caloric restriction. The materials may also be administered for the purpose of increasing muscle mass concurrently with a high protein diet as well as with an exercise program.
Owner:ADVANTRA Z INC
Nutritional intervention for improving muscular function and strength
ActiveUS9259430B2Increasing muscle mass and strength and functionalityImprove functionalityVitamin food ingredientsAnimal feeding stuffMuscle functionsUltimate tensile strength
The present invention provides a composition comprising HMB and Vitamin D. Methods of administering HMB and Vitamin D to an animal are also described. Vitamin D and HMB are administered to increase muscle mass, strength, and functionality. The combination of Vitamin D and HMB together has a synergistic effect, which results in a surprising and unexpected level of improvement in muscle mass, strength and functionality.
Owner:METABOLIC TECH
ACE-2 modulating compounds and methods of use thereof
InactiveUS7045532B2Reduce appetiteAppetite of the subject is decreasedBiocidePeptide/protein ingredientsDiseaseAmino acid
ACE-2 inhibitors for the treatment of body weight and related disorders are disclosed. ACE-2 inhibitors include peptides and small molecules. Examples of small molecule ACE-2 inhibitors include compounds of formula (I):Z-Λ (I)Wherein Z is a zinc coordinating moiety and Λ is an amino-acid mimicking moiety, and pharmaceutically acceptabale salts thereof. Methods of using the inhibitors and pharmaceutical compositions containing the inhibitors to treat a body weight disorder, to decrease appetite, to increase muscle mass, to decrease body fat, to treat diabetes and to treat a state associated with altered lipid metabolism, are also described.
Owner:MILLENNIUM PHARMA INC
Anti-myostatin antibodies, polypeptides containing variant Fc regions, and methods of use
ActiveUS10738111B2Quality improvementHigh strengthMuscular disorderImmunoglobulins against growth factorsImmunologic disordersMyostatin
The disclosure provides anti-myostatin antibodies and methods of making and using the same. Nucleic acids encoding the anti-myostatin antibodies and host cells comprising the nucleic acids are also provided. The anti-myostatin antibodies have uses that include treating a muscle wasting disease, reducing body fat accumulation, and increasing mass and strength of muscle tissue. The disclosure also provides polypeptides containing a variant Fc region and methods of making and using the same. Nucleic acids encoding the polypeptides and host cells comprising the nucleic acids are also provided. The polypeptides have uses that include suppressing the activation of immune cells; treating an immunological inflammatory disease, autoimmune disease, or viral infection; and increasing muscle mass and strength or reducing body fat accumulation.
Owner:CHUGAI PHARMA CO LTD
System and method for skeletal muscle stimulation
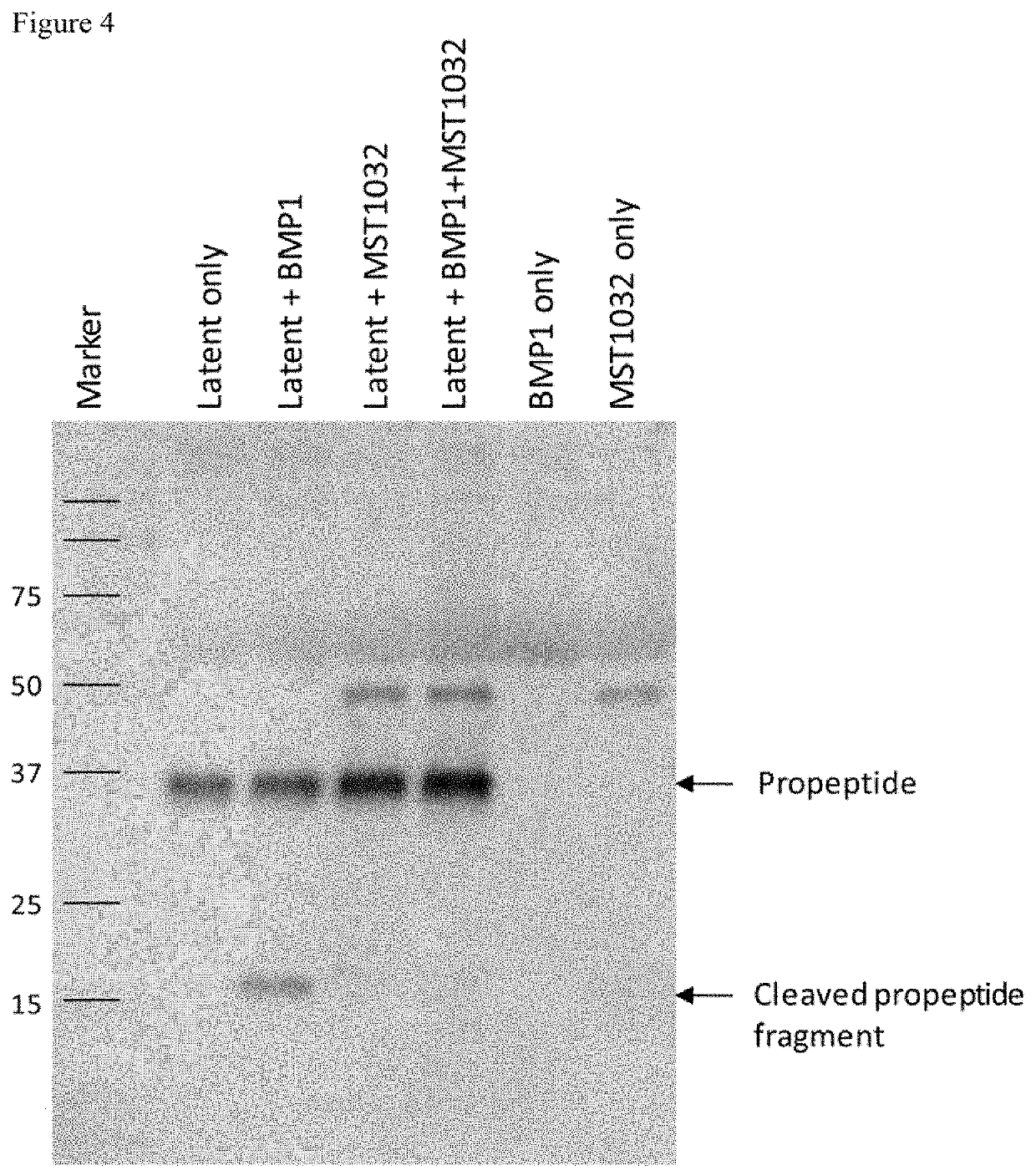
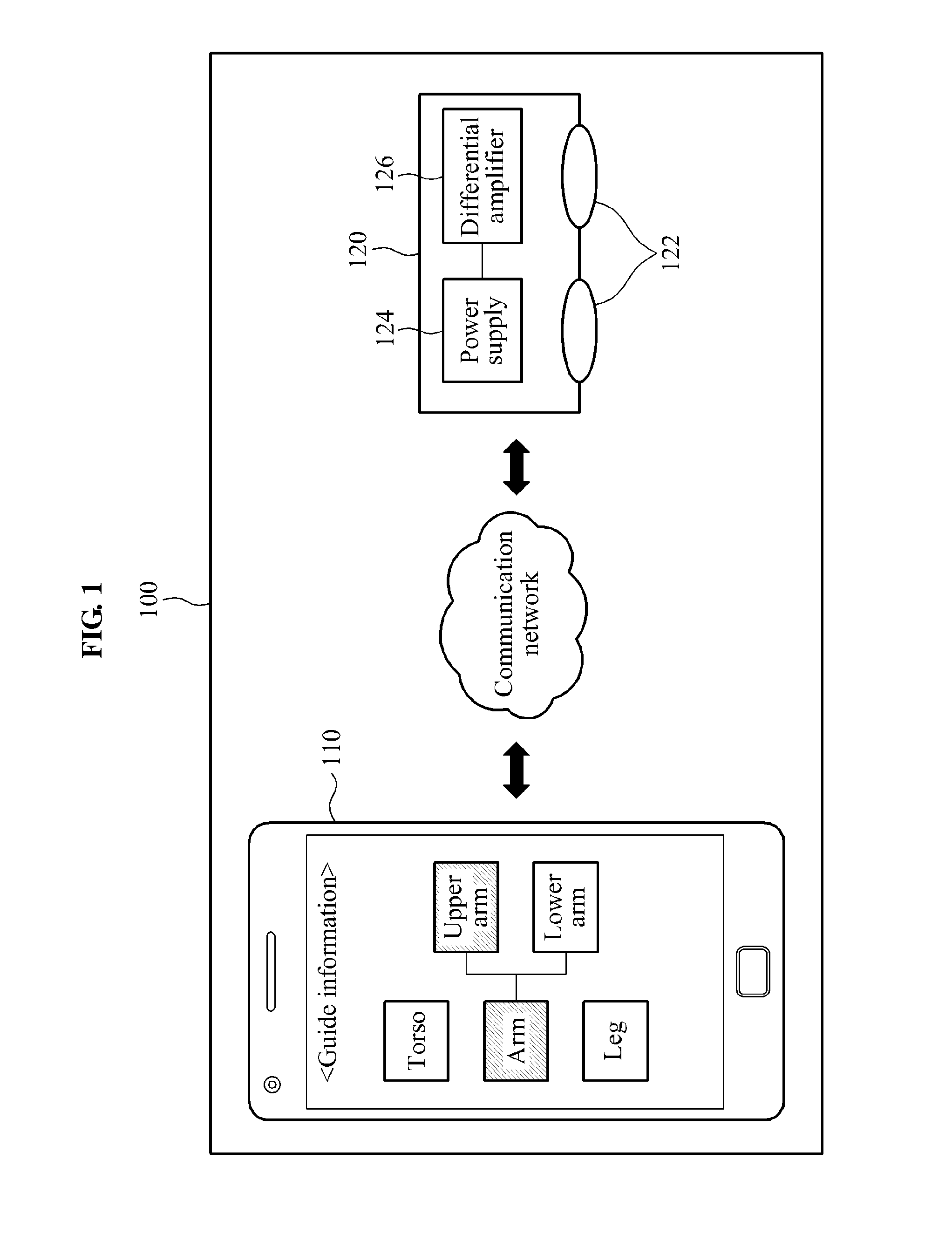
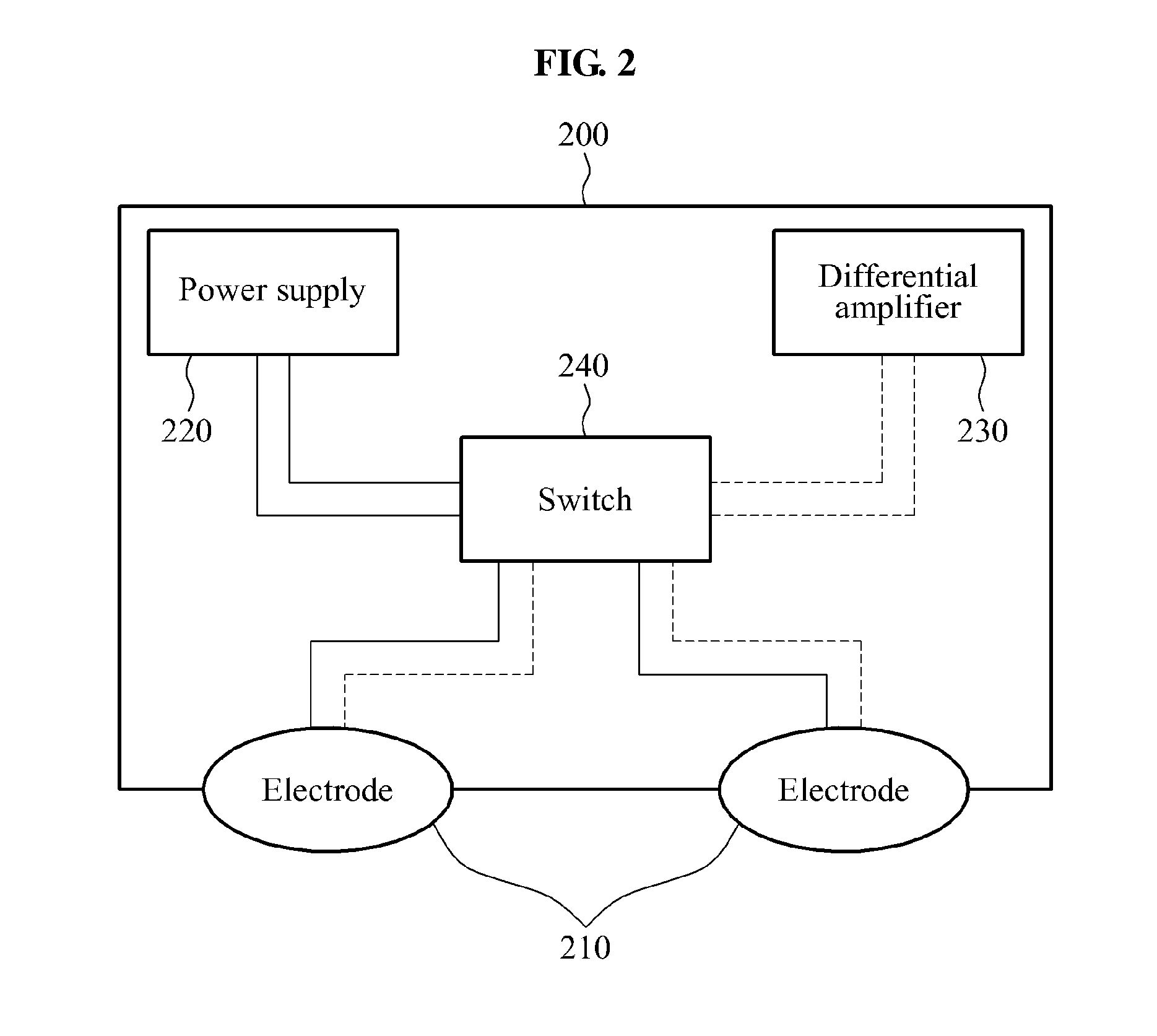
ActiveUS20140188188A1Fail to satisfyElectrotherapyElectromyographyPhysical medicine and rehabilitationSkeletal muscle
Owner:SAMSUNG ELECTRONICS CO LTD
Anti-myostatin antibodies
Anti-myostatin antibodies are identified that are characterized as having high affinity and may be chimeric, humanized or fully human antibodies, immunoconjugates of the antibodies or antigen-binding fragments thereof. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment of various disorders in mammalian and avian species.
Owner:ELI LILLY & CO
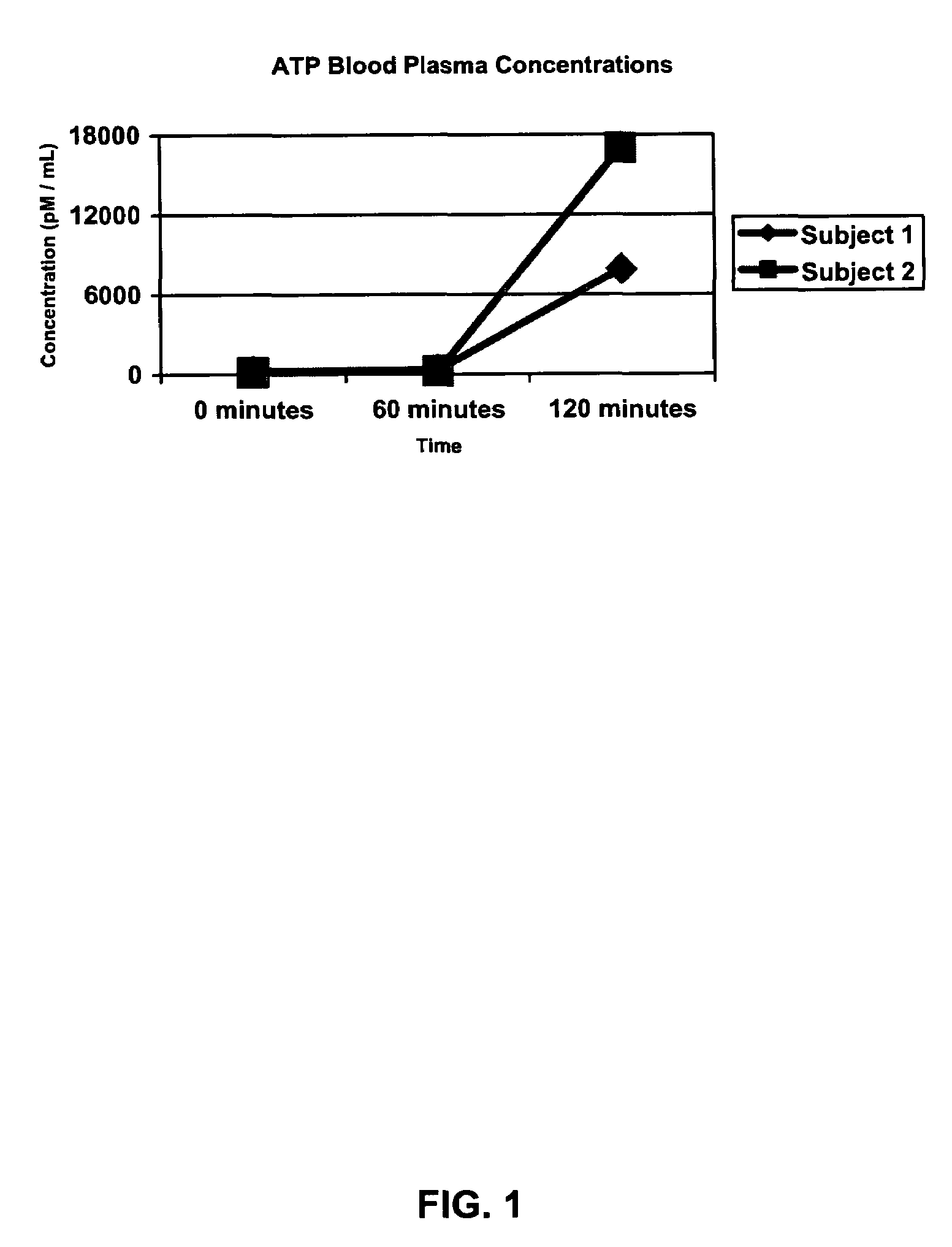
Method for increasing muscle mass and strength through administration of adenosine triphosphate
ActiveUS7629329B2Promote absorptionAdd additional massBiocideCarbohydrate active ingredientsNucleotideGastric juices
The present invention is directed to compositions having an effective amount of Adenosine Triphosphate (“ATP”) sufficient to effect intracellular and extracellular concentrations of ATP in a mammal to improve anaerobic exercise capacity by increasing muscle size and / or strength and methods for using the same. Preferably, a gastric acid secretion inhibitory coating is applied to the effective amount of ATP in a manner that protects the ATP from degradation by gastric juices. ATP compositions of the present invention may be administered in nutraceutical or functional food dosage forms, including oral and non-oral delivery forms. In addition, the effective amount of ATP maybe combined with amino acids, botanicals, functional foods, herbals, nucleotides, nutraceuticals, nutrients, pharmaceuticals, proteins, and / or vitamins in an effort to enhance the targeted activity of the composition.
Owner:TSI GRP CO LTD
Method for down-regulating GDF-8 activity
Disclosed are novel methods for increasing muscle mass by means of immunization against Growth Differentiation Factor 8 (GDF-8, myostatin). Immunization is preferably effected by administration of analogues of GDF-8 which are capable of inducing antibody production against homologous GDF-8. Especially preferred as an immunogen is homologous GDF-8 which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes while substantially preserving the tertiary structure of the homologous GDF-8. Also disclosed are nucleic acid vaccination against GDF-8 and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for identification of useful immunogenic GDF-8 analogues, methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:PHARMEXA
Methods for increasing the muscle mass of a human with materials derived from citrus varieties
Materials derived from Citrus plants can be administered orally to humans for the purpose of producing or maintaining weight loss as well as for improving the person's physical performance and increasing the person's lean muscle mass. The Citrus materials include those portions of the plant that are normally considered waste or inedible, such as the leaves, peel, and immature, unripe fruit. The materials contain at least one of the alkaloids from the group consisting of synephrine, hordenine, octopamine, tyramine and N-methyltyramine (1). Two species, Citrus aurantium and Citrus reticulata, are particularly useful. The materials can be administered in their natural form or as extracts, and can be administered in various ways including capsules and tablets. The Citrus materials may also be used as a tea. For weight loss and weight control, the materials can be administered concurrently with caloric restriction or in the absence of caloric restriction. The materials may also be administered for the purpose of increasing muscle mass concurrently with a high protein diet as well as with an exercise program.
Owner:ADVANTRA Z INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com