Chimeric receptor genes and cells transformed therewith
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Constructions and Expression of the Chimeric ScFvRγ / ζ chain genes
[0077]In this example, the following materials and methods were used.
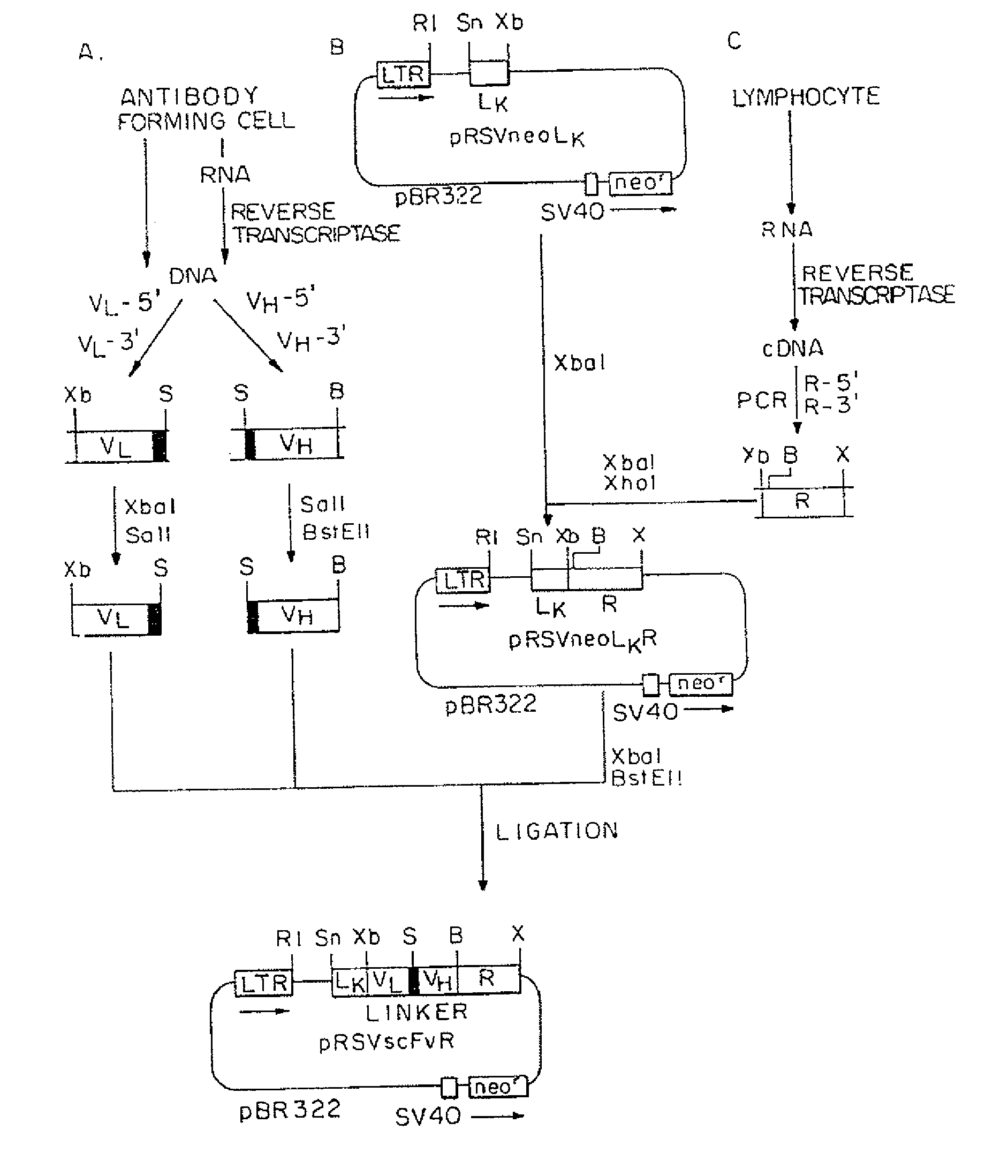
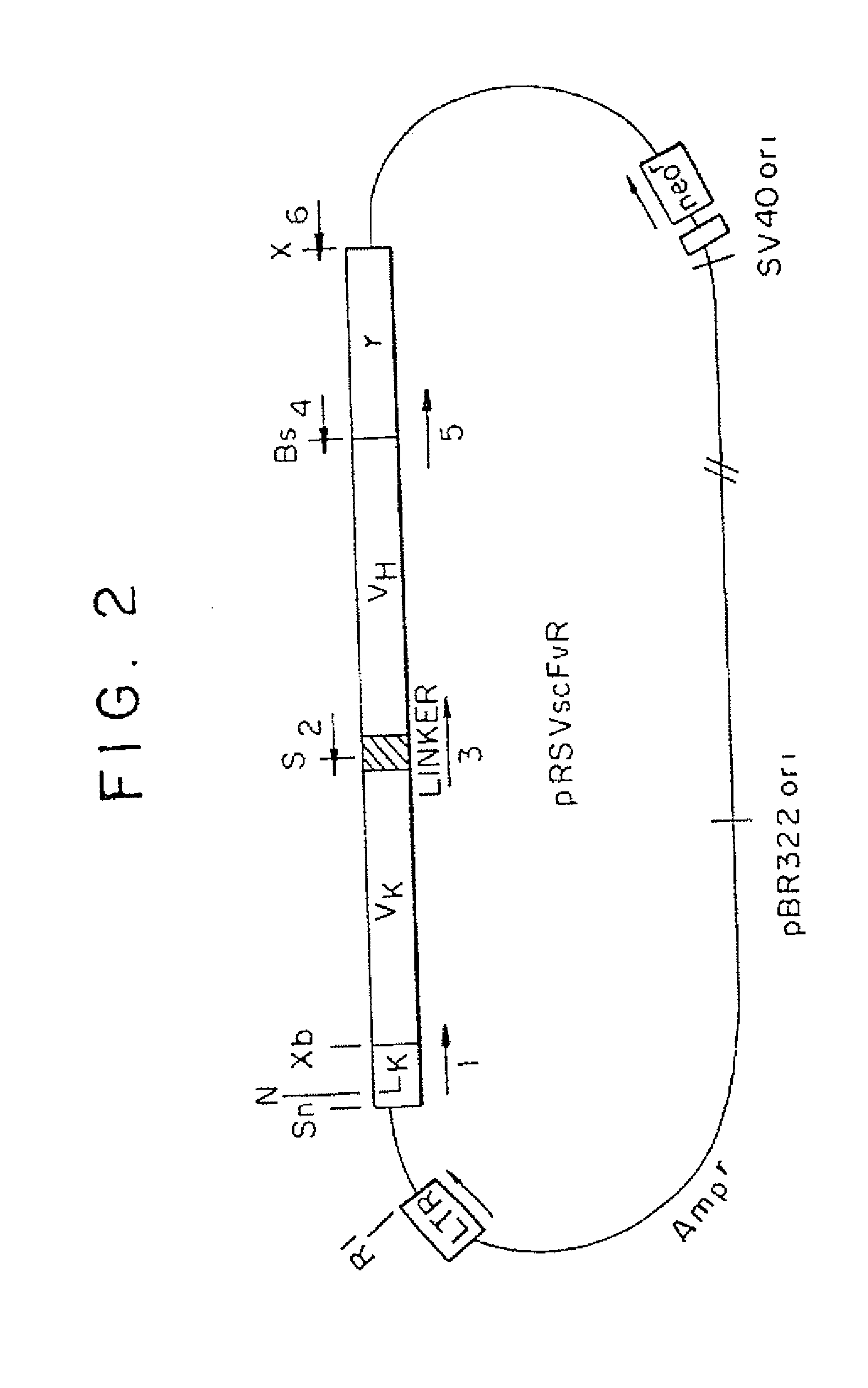
[0078]A. Cell lines and antibodies. MD.45 is a cytolytic T-lymphocyte (CTL) hybridoma of BALB / c mice allospecific to H-2b (29). MD45.27J is a TCR α-mutant of MD.45. A.20 is a B lymphoma of BALB / c origin (ATCC#T1B 208). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Sp6, an anti-TNP mAb, and 20.5, an anti-Sp6 idiotype mAb, were provided by G. Kohler (30). Anti-human FcεRIγ chain polyclonal and monoclonal (4D8) (31) antibodies were provided by J.-P. Kinet and J. Kochan, respectively, and rabbit antibodies to murine zeta chain by M. Baniyash.
[0079]B. Constructions of chimeric genes. All the recombinant DNA manipulations were carried out as described in updated editions of Sambrook et al. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, N.Y., and Ausubel et al. (1987) Curren...
example 2
Expression of scFvRγ / ζ as Functional Receptors
[0085]To test whether the chimeric scFvRγ or scFvζ can function as an active receptor molecule, we studied the ability of the transfected hybridomas to undergo antigen-specific stimulation. The MD.45 T cell hybridoma can be triggered through its TCR to produce IL-2, IL-3 or GM-CSF. It specifically recognizes and responds to H-2b target cells (29), while its MD45.27J mutant cannot be stimulated through its TCR due to the absence of an α chain. Upon introduction of the chimeric Sp6-scFvRγ, both of these cells could be specifically triggered to produce IL-2 following incubation with TNP-modified stimulator cells (FIG. 6A) or plastic-immobilized TNP-fowl gamma globulin (TNP-FγG) (FIG. 6B). Non-modified A.20 cells or FγG did not activate the transfectants, demonstrating the specificity of the response toward TNP. Stimulation of the various transfectants with immobilized antigen resulted in different degrees, of reactivity. While STA responded...
example 3
Targeting of Cytolytic Lymphocytes to Neu / HER2 Expressing Cells Using Chimeric Single-Chain Fv Receptors
[0090]Cell surface molecules essential for the transformed phenotype or growth of malignant cells are attractive targets for anti-cancer immunotherapy. Antibodies specific to Neu / HER2, a human adenocarcinoma-associated growth factor receptor, were demonstrated to have tumor inhibitory capacity. Yet, the inefficient accessibility of antibodies to solid tumor limits their clinical use. To redirect effector lymphocytes to adenocarcinomas, we constructed and functionally expressed in T cells chimeric single-chain receptor genes incorporating both the antigen binding domain of anti-Neu / HER2 antibodies and the γ or ζ signal transducing subunits of the T cell receptor / CD3 and the immunoglobulin Fc receptor complexes. Surface expression of the anti-Neu / HER2 chimeric genes in cytotoxic T cell hybridomas endowed them with specific Neu / HER2 recognition enabling their activation for interleuk...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com