Isolated population of plant single cells and method of preparing same
a single cell and plant technology, applied in the field of plant single cell population and isolating population, can solve the problems of low productivity of plant cell culture for secondary metabolite production, slow growth rate, and variability still a major issue, and achieve stable production of biologically active substances, less change in cell growth rate, and less sensitive to shear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Practical Example 1
Preparation of Plant Materials and Isolation of Cambium
[0053]Seed, needle, twig of the yew tree were collected. After collecting the materials, they were deposited in the solution of 100 mg / L of antioxidant, ascorbic acid (L-ascorbic acid, DUCHEFA, The Netherlands) immediately and transferred and preserved. They were surface sterilized by considering the morphology and physiological characteristics of the materials.
[0054]1. Seed: After sterilizing the seeds with 70% ethanol for one minute, they were immersed in 1% Clorox solution for 48 hours and were washed 3 to 4 times with sterile water. Next, embryo was separated from the seed in the solution of 0.5% PVP (polyvinyl pyrrolidone, DUCHEFA, The Netherlands) and 50 mg / L of ascorbic acid (L-ascorbic acid, DUCHEFA, The Netherlands), and 70 mg / L of citric acid (DUCHEFA, The Netherlands) and cultured on the callus induction media.
[0055]2. Needle and twig: After 24 hours of treatment with the solution containing 1% Beno...
example 2
Practical Example 2
Induction of Single Cell Clone from the Isolated Cambium
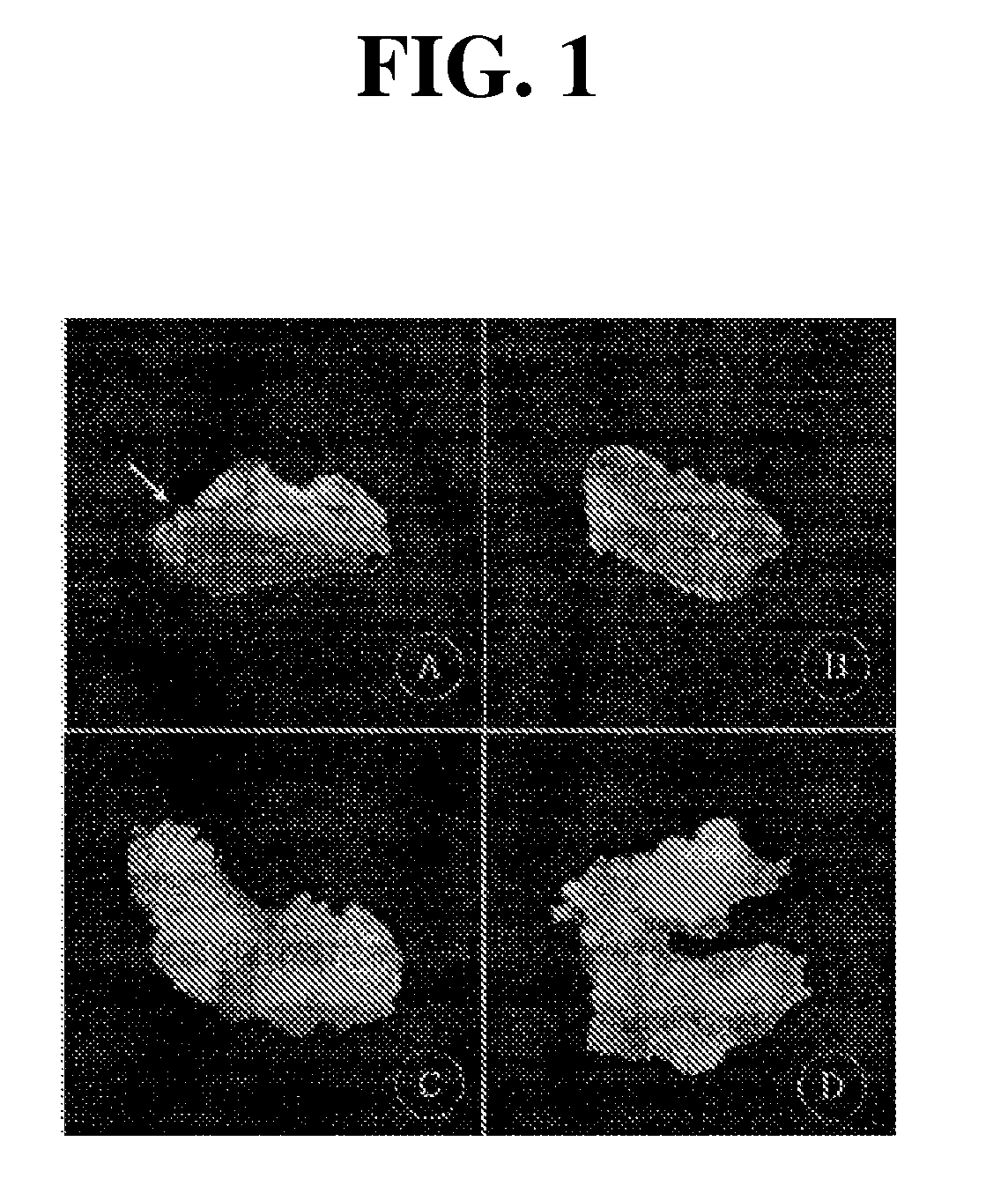
[0057]After 4th to 7th day of the culture, cell division of cambium was observed and on the 15th day of the culture, callus was beginning to form from the layer consisted of the phloem and cortex and epidermis that were the upper part of the cambium. On the 30th day of the culture, the cambium began to be separated from the upper layer tissue that contained the phloem and cortex and epidermis; after these two layers were completely separated naturally, they were cultured individually on different petri dishes (FIG. 1).
[0058]For the purpose of cell and callus induction, universally known media of the plant cell and tissue culture could be used: e.g. mB5 (modified Gamberg's B5 medium), MS (Murashige & Skoog medium), WPM (Lloyed & McCown), SM (schenk & Hildebrand medium), LP (Quoirin & Lepiovre). Application of all these media is possible. Various additives could be supplemented and components of the media could...
example 3
Practical Example 3
Establishment of Long Term Culture
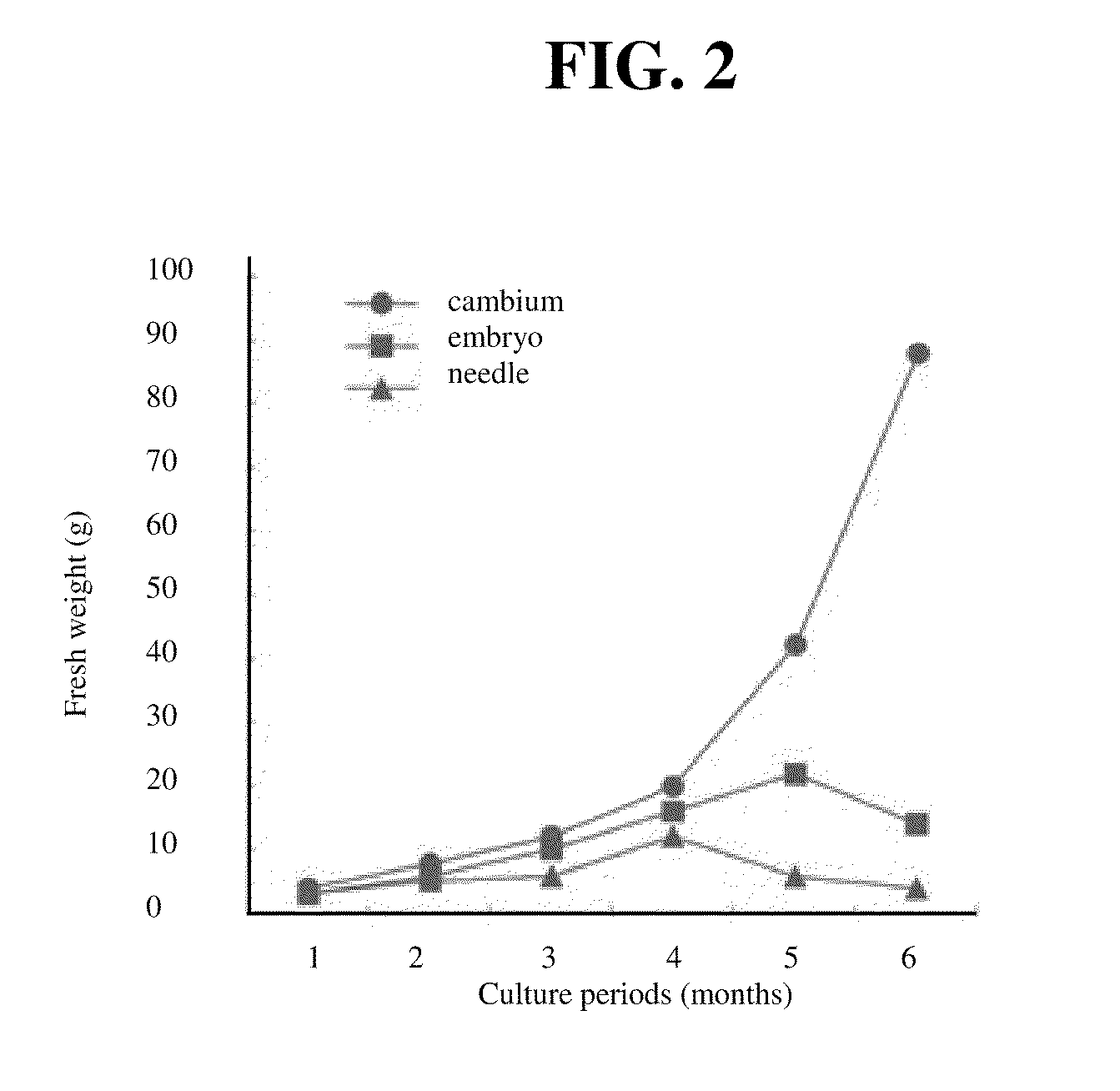
[0062]Among the calli, white and friable calli that had good growth rate were subcultured onto the new media every 21 days. The growth rate of the embryo and needle-derived cultures was very unstable and it often showed the tendency of browning. On the contrary, the growth rate of the cambium-derived cultures was fast and there was no color change of the cultures. Therefore, it was possible to select the stable cells.
[0063]After six months of the culture, most of the embryo and needle-derived cultures had yellow or light brown color and aggregation formed. The cambium-derived cultures had white-yellow color and were maintained as single cells or small cell clusters. The growth rate of the cultures that turned brown and formed aggregation slowed down and the cultures died eventually because of the phenol chemical substance that they excreted.
[0064]According to this inventor, maintenance and mass proliferation of the embryo and need...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com