Methods for treatment of metabolic disorders using epimetabolic shifters, multidimensional intracellular molecules, or environmental influencers
a metabolic disorder and epimetabolic shifter technology, applied in the field of metabolic disorders treatment, prevention and reduction, can solve the problems of short serum half-life of insulin, major impediment to the maintenance of normoglycemia, and limited success of strategy, so as to achieve the effect of effectively treating or preventing the progression of metabolic diseases, restoring or promoting more normal mitochondrial osidative phosphorylation, and maintaining normal mitochondrial function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of CoQ10 as a MIM
[0295]In order to evaluate CoQ10 as a potential MIM, CoQ10 in oxidized form was exogenously added to a panel of cell lines, including both cancer cell lines and normal control cell lines, and the changes induced to the cellular microenvironment profile for each cell line in the panel were assessed. Changes to cell morphology / physiology, and to cell composition, including both mRNA and protein levels, were evaluated and compared for the diseased cells as compared to normal cells. The results of these experiments identified CoQ10 and, in particular, the oxidized form of CoQ10, as a MIM.
[0296]In a first set of experiments, changes to cell morphology / physiology were evaluated by examining the sensitivity and apoptotic response of cells to CoQ10. A panel of skin cell lines including a control cell lines (primary culture of keratinocytes and melanocytes) and several skin cancers cell lines (SK-MEL-28, a non-metastatic skin melanoma; SK-MEL-2, a metastatic s...
example 2
Methods for Identifying Disease Relevant Processes and Biomarkers for Metabolic Disorders
[0300]From the cell based assays in which cell lines were treated with a molecule of interest, the differences in treated vs non-treated cells is evaluated by mRNA arrays, protein antibody arrays, and 2D gel electrophoresis. The proteins identified from comparative sample analysis to be modulated by the MIM or Epi-shifter, are evaluated from a Systems Biology perspective with pathway analysis (Ingenuity IPA software) and a review of the known literature. Proteins identified as potential therapeutic or biomarker targets are submitted to confirmatory assays such as Western blot analysis, siRNA knock-down, or recombinant protein production and characterization methods.
Materials and Methods for Examples 3-8
[0301]Coenzyme Q10 stock
[0302]A 500 μM Coenzyme Q10 (5% isopropanol in cell growth media) was prepared as follows. A 10 mL 500 μM Coenzyme Q10 stock was made fresh every time.
Molecular Weight: 863...
example 3
Sensitivity of Cell Lines to CoQ10
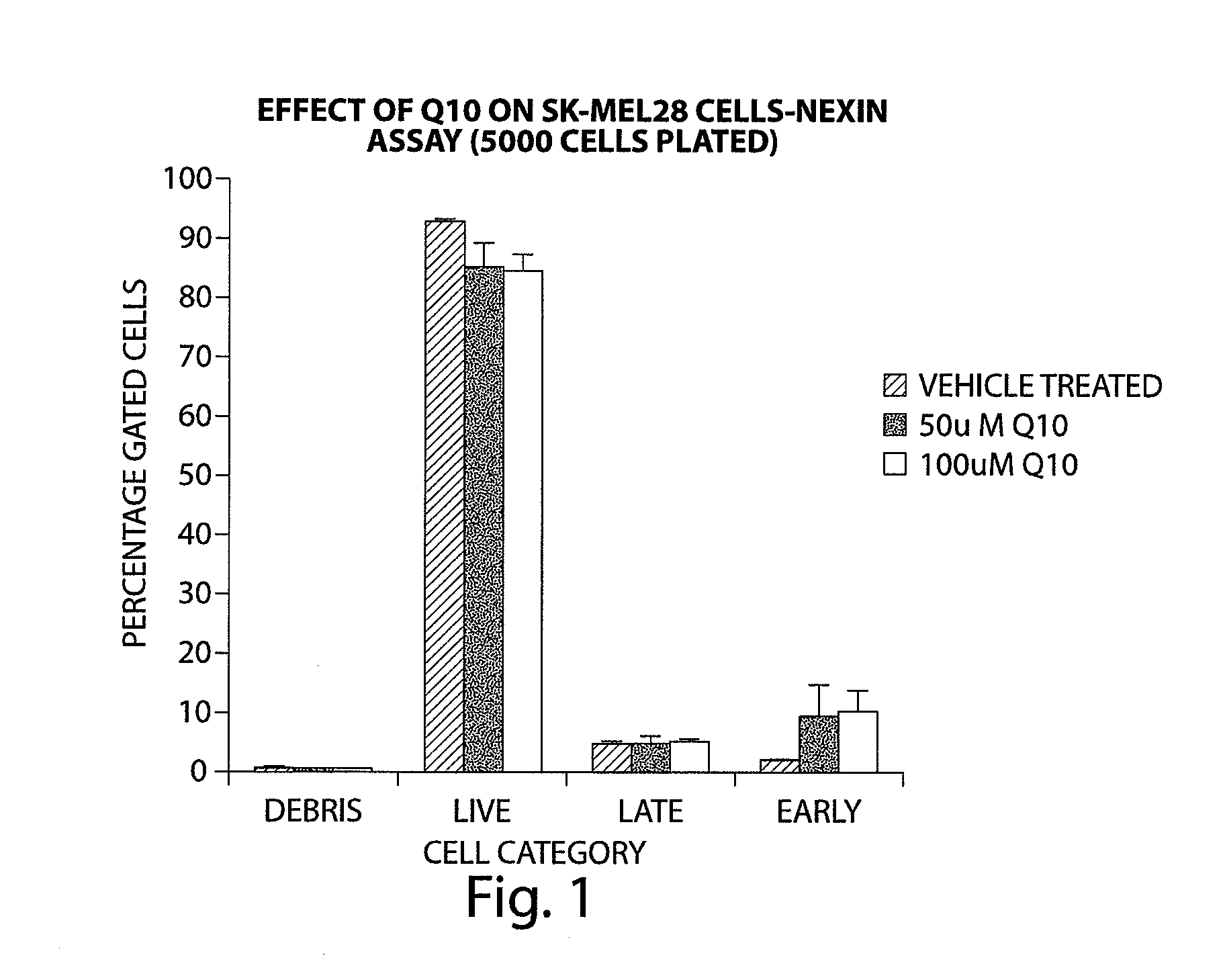
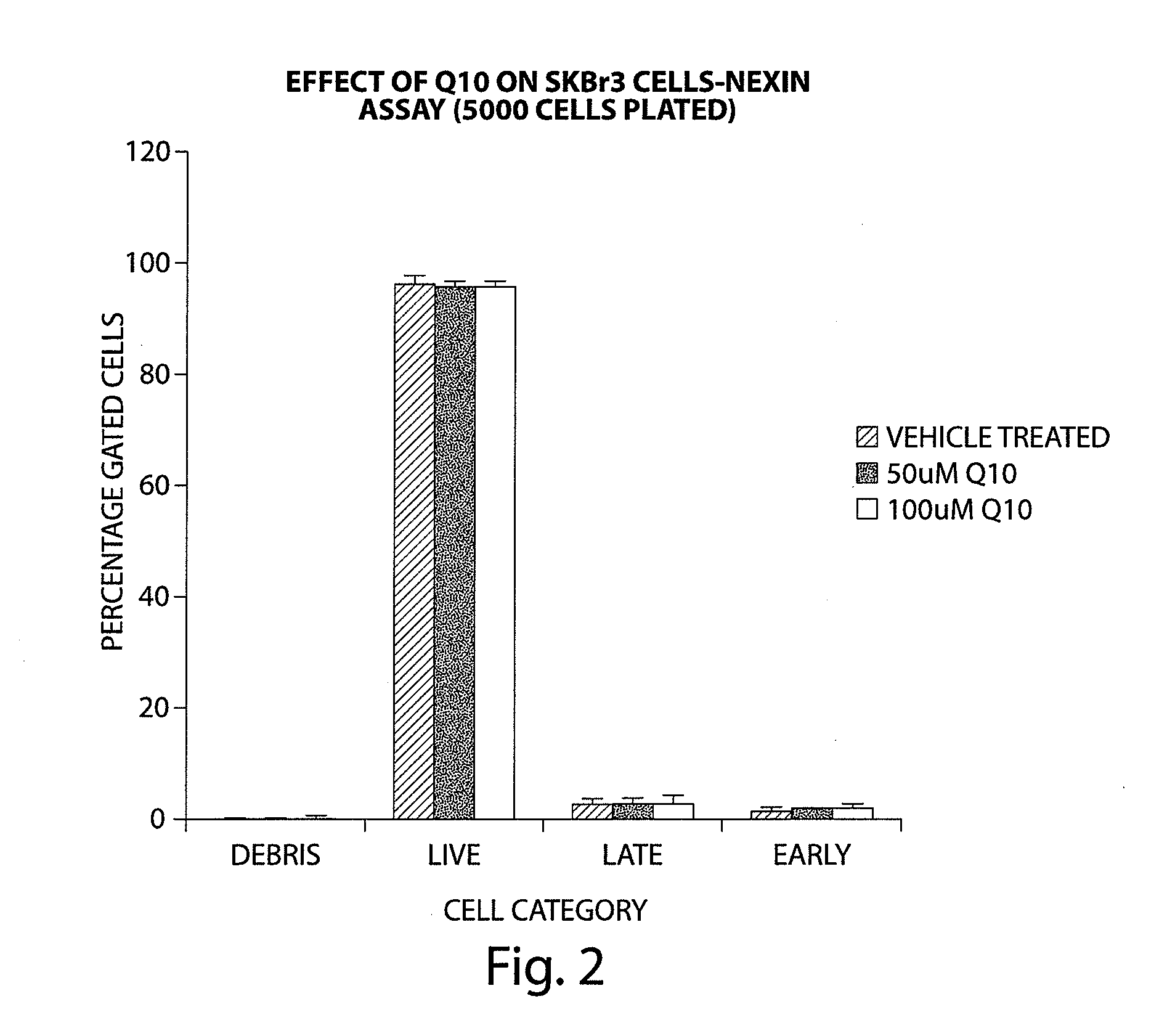
[0328]A number of cell lines were tested for their sensitivity to Q10 after 24 hours of application by using a reagent (Nexin reagent) that contains a combination of two dyes, 7AAD and Annexin-V-PE. The 7AAD dye will enter into cells with permeabilized cell membranes; primarily those cells that are in late apoptosis. Annexin-V-PE is a dye that binds to Phosphotidyl serine, which is exposed on the outer surface of the plasma membrane in early apoptotic cells. The Nexin reagent thus can be used to differentiate between different populations of apoptotic cells in a flow cytometer.
[0329]PaCa2 cells showed an increase in both early and late apoptotic cells (between 5-10% of gated cells) with 50 μM Q10 and 100 μM Q10 after 24 hours of Q10 application. PC-3 cells also showed an increase in both early and late apoptotic population with 50 μM and 100 μM Q10, although the increase was less when compared to PaCa2 cells. MCF-7 and SK-MEL28 cells showed an incre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com