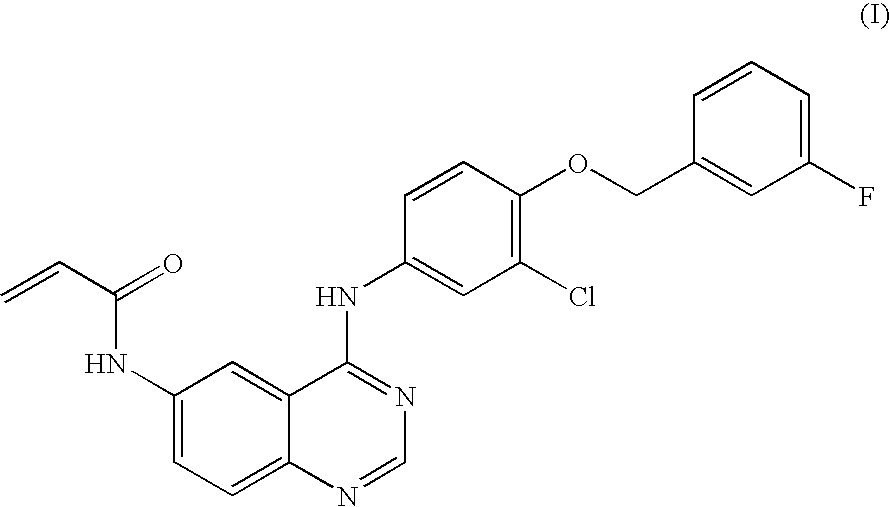
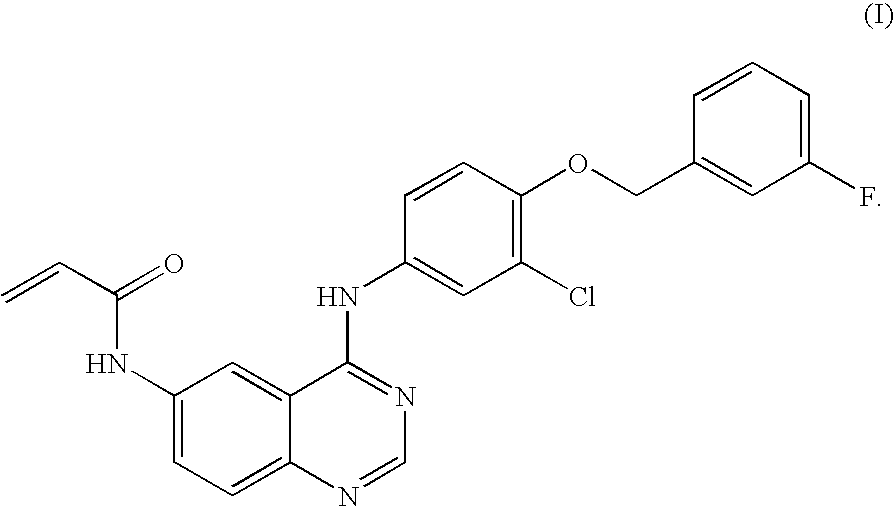
Salts of 4-aniline quinazoline derivative
a quinazoline and 4-aniline technology, applied in the field of salts of 4phenylamino quinazoline derivatives, can solve the problems of excessive or deficient production of some proteins, tumor cells and cancer, excessive cell proliferation, etc., and achieve excellent tumor inhibitory activity, low toxicity, and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]quinazolin-6-yl}-acrylamide
Step A: The preparation of 4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-6-nitro-quinazoline
[0046]1) 2.85 g (15 mmol) of 6-nitro-quinazolone and 25 ml of phosphorus oxychloride were added to a flask of 100 ml equipped with a reflux condenser and then refluxed for 3 hours at 105° C., which was then poured carefully into ice-water system of 150 ml. The squamose solid precipitated slowly. The solid was collected by filtration and dried, and was then identified as 4-chloro-6-nitro-quinazoline with a yield of 78%.
[0047]1H-NMR (400 MHz, CDCl3): δ9.22 (2H, s), 8.74 (1H, dd, J=2.57 Hz, 9.16 Hz), 8.27 (1H, d, J=9.16 Hz).
[0048]2) 4.65 g (26.6 mmol) of 2-chloro-4-nitrophenol, 3.31 ml (27.0 mmol, 1 eq) of m-fluorobenzyl bromide, 9.4 g (54 mmol, 2 eq) of potassium carbonate and 50 ml of dimethyl formamide were added to a flask of 250 ml equipped with a reflux condenser, and then heated and refluxed. The solid was remove...
example 2
The hydrochloride of N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenyl-amino]-quinazolin-6-yl}-acrylamide
[0056]1.0 g (2.23 mmol) of the compound N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenyl-amino]-quinazolin-6-yl}-acrylamide prepared according to Example 1 was dissolved in 20 mL of the mixture of ethyl acetate and triethylamine (EA / Et3N=40 / 1). The solution was stirred in an ice-water bath and thereto, 2 mL of the solution of HCl in 1,4-dioxane (4 mol / L) was added dropwise slowly. Some yellow solid precipitated, and the stir was stopped after 45 min. The solid was collected by filtration, washed with water and dried to obtain 530 mg (1.09 mmol) of Kelly solid, which was identified as the hydrochloride of N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-quinazolin-6-yl}-acryl amide with a yield of 49%. MS: 449. mp: 249-252°.
[0057]1H-NMR (400 MHz, CDCl3+DMSO): δ8.91 (1H, s), 8.76-8.69 (2H, m), 8.01 (1H, d), 7.83 (2H, s), 7.68 (1H, dd), 7.46-7.33 (2H, m), 7.34-7.29 (2H, m), 7.23-7.18 (2H, m),...
example 3
The sulfate of N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-quinazolin-6-yl}-acrylamide
[0059]According to the method described in Example 2, the solution of sulfuric acid in 1,4-dioxone (2 mol / L) was used instead of the solution of HCl in 1,4-dioxane (4 mol / L).
[0060]The sulfate of N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-quinazolin-6-yl}-acrylamide was obtained.
[0061]1H-NMR (400 MHz, CDCl3+DMSO): δ8.99 (1H, s), 8.82-8.76 (2H, m), 8.10 (1H, d), 7.90 (2H, s), 7.74 (1H, dd), 7.53-7.40 (2H, m), 7.42-7.37 (2H, m), 7.31-7.26 (2H, m), 6.60 (2H, d), 6.35 (1H, t), 5.70 (2H, s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com