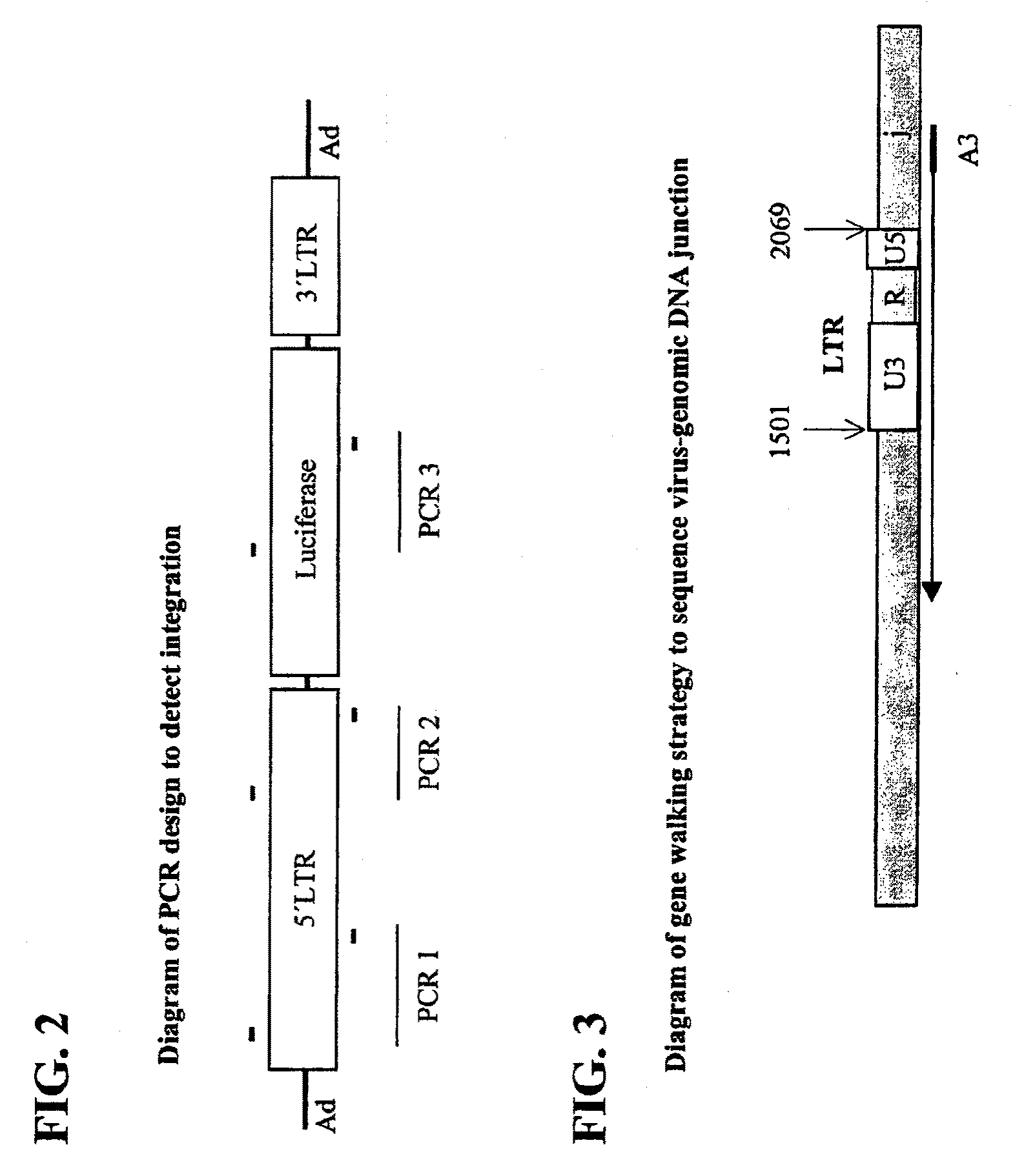
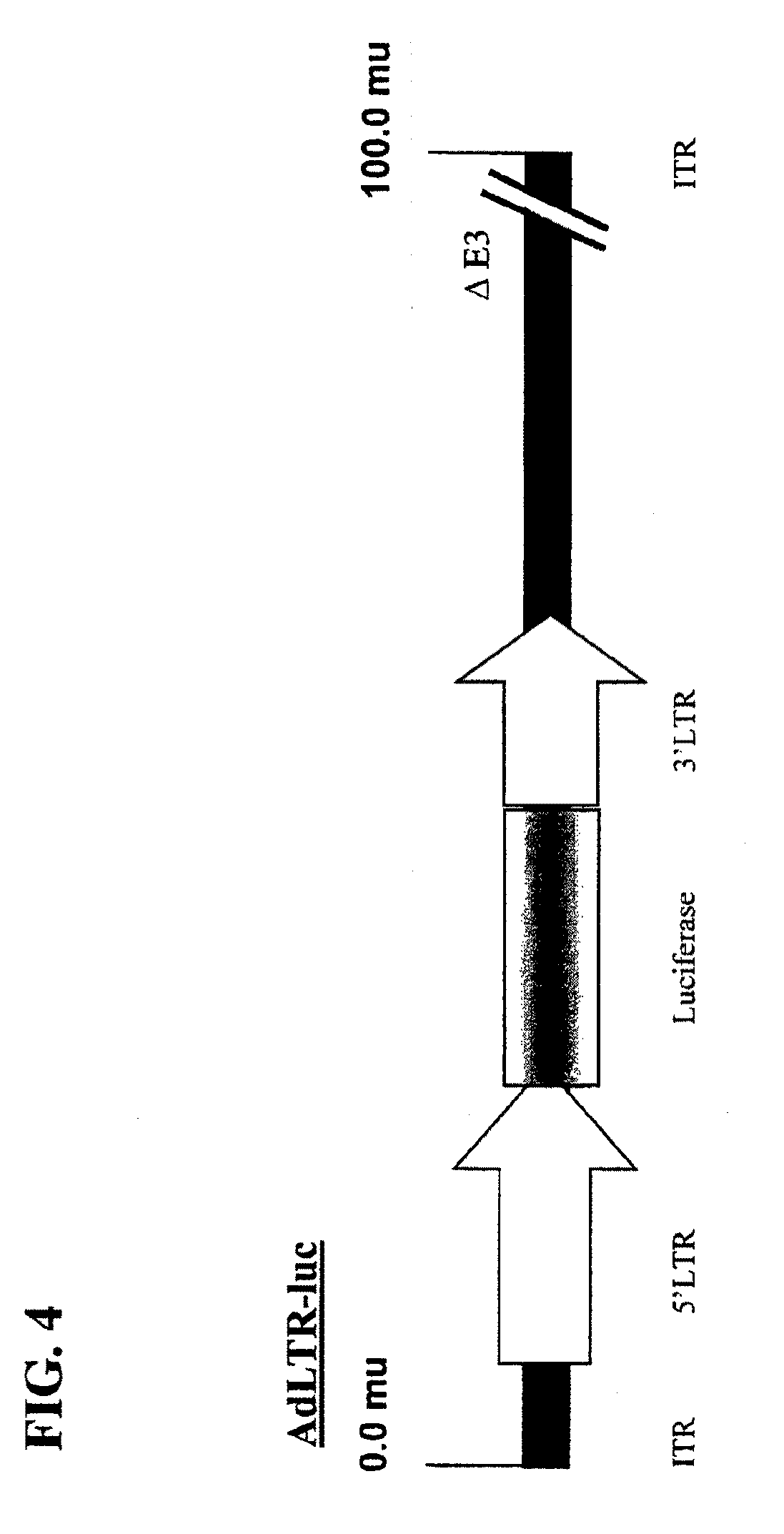
Hybrid adeno-retroviral vector for the transfection of cells
a technology of adenovirus and adenovirus, which is applied in the direction of viruses/bacteriophages, drug compositions, dsdna viruses, etc., can solve the problems of inability to transfer nucleic acids into cells with high efficiency, neither of these methods allow for persistent gene expression, and achieve the effect of meditating long-term gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Recombinant Viral Vectors
[0114]The replication-deficient recombinant adenoviral vectors used are based on the adenovirus type 5 (Ad5) genome (see Becker et al., 1994, “Use of Recombinant Adenovirus for Metabolic Engineering of Mammalian Cells,” in: Method in Cell Biology 43:161-189, herein incorporated by reference). E1 deletion was achieved by recombination of the pAC shuttle plasmid with pJM17. 2.7 kb or pBHG10 (Microbix Biosystems; Ontario, Calif.) of 5′LTR (which includes part of the envelope gene [1.5 kb], the 5′LTR [0.57 kb], and the packaging sequence [0.63 kb]) and 1 kb of 3′LTR (which contains a small part (˜0.5 kb) of the envelope gene and an intact 3′LTR). A restriction map of the 5′ LTR is shown in FIG. 10. A restriction map of the 3′ LTR is shown in FIG. 11.
[0115]MoMLV sequences were cleaved by EcoR I from the plasmid pXT1 (Stratagene, Catalog number 214201, La Jolla, Calif.) (Boulter and Wagner, Nucleic Acids Res. 15: 7194, 1987). Not I linkers were add...
example 2
[0116]Cell culture: The human mononuclear cells and macrophages were obtained from the peripheral blood of normal volunteers. The cells were separated on Ficoll Hypaque, and washed twice with PBS. The mononuclear cells were cultured in suspension in RPMI 1640 with 10% human serum for 2 weeks before infection. The macrophages were adherent to the bottom of the flask after the mononuclear cells from peripheral blood were cultured for a week. The supernatant was replaced by fresh growth medium twice a week for 25 days before infection.
[0117]Hippocampus neurons (Dr. Z. G. Jiang, MH, NIH, Bethesda, Md.), were obtained from Tac:N(SD)fBR rats at 18 gestational days. Hippocampus tissue was cut into 1 mm cubes and then triturated by using fire-restricted Pasteur pipettes to achieve single cells. The cells were seeded at a density of 40,000 / well in a 96-well plate, and cultured in neurobasal medium supplemented with 1×B27 and 2 mM glutamine for two weeks before infection. ...
example 3
Gene Expression In Vitro and In Vivo
[0127]The salivary epithelial cells, HSY, A5 and HSG, grow readily in vitro. Human mononuclear cells and macrophages, and rat hippocampus neurons were cultured without cell proliferation. All of these cell types were readily infected by AdCMV-luc and AdLTR-luc (FIG. 1A, 1B).
[0128]In order to assess the time course of AdLTR-luc and AdCMV-luc persistence in vivo, rat submandibular glands were infected locally by retrograde ductal instillation of 1×109 pfu / gland. In submandibular glands the levels of luciferase activity dropped quickly in both virus groups during the two weeks following infection (FIG. 1C). The levels of luciferase activity were then maintained in the AdLTR-luc infected animals until 9 weeks (the last time point studied) while they continued to decline in the AdCMV-luc group. At the 9 week time point average luciferase activity in glands administered AdLTR-luc was 15-fold greater than in glands administrated AdCMV-luc (9.1 RLU / 25 μg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com