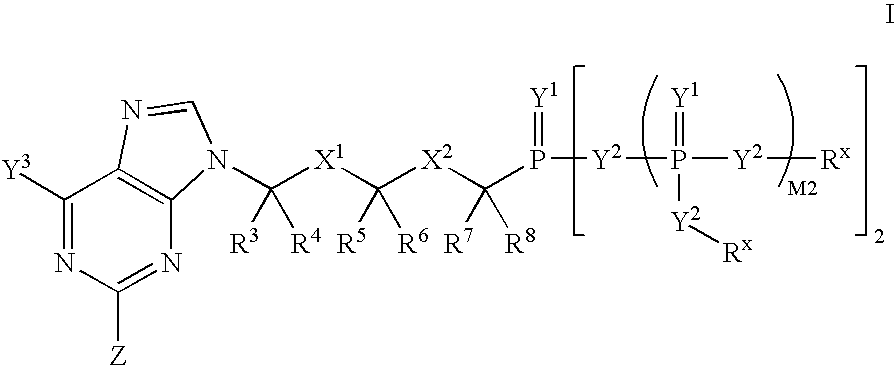
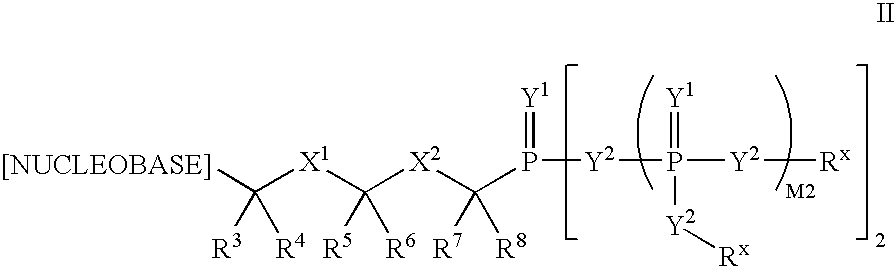
Nucleobase phosphonate analogs for antiviral treatment
a technology of nucleotide phosphonate and antiviral treatment, which is applied in the direction of biocide, group 5/15 element organic compounds, drug compositions, etc., can solve the problems and affecting the treatment effect of patients, so as to improve the therapeutic and diagnostic value, the effect of increasing the accumulation and retention of drug compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Section A
[0283]
[0284] Scheme A shows the general interconversions of certain phosphonate compounds: acids —P(O)(OH)2; mono-esters —P(O)(OR1)(OH); and diesters —P(O)(OR1)2 in which the R1 groups are independently selected, and defined herein before, and the phosphorus is attached through a carbon moiety (link, i.e. linker), which is attached to the rest of the molecule, e.g. drug or drug intermediate (R). The R1 groups attached to the phosphonate esters in Scheme 1 may be changed using established chemical transformations. The interconversions may be carried out in the precursor compounds or the final products using the methods described below. The methods employed for a given phosphonate transformation depend on the nature of the substituent R1. The preparation and hydrolysis of phosphonate esters is described in Organic Phosphorus Compounds, G. M. Kosolapoff, L. Maeir, eds, Wiley, 1976, p. 9ff.
[0285] The conversion of a phosphonate diester 27.1 into the corresponding phosphonate ...
preparation 1
Synthesis of SDS-1 S-(+)-N-Propenoylbornane-2,10-sultam
[0355] The acrylic acid (7.04 mL, 1.2 eq.) bought from Aldrich was dissolved in 120 ml of tetrahydrofuran (THF), 27.5 mL of triethyl amine (TEA) was added thereto and the resulting mixture was cooled to −20° C. To mixture 11.66 mL (1.2 eq.) of pivial chloride was added and stirred for 1 hour. To the reaction mixture add lithium chloride (3.7 g, 1.1 eq.) and bornane 2,10 sultam (17 g, 1 eq.), allow the mixture to warm to RT and stir another 4-6 hours. Reaction mixture was quenched with 10 mL 0.2 M hydrochloric acid (2 eq.). The mixture was reduced under vacuum to remove THF. Extract the aqueous mixture with ethyl acetate (EtOAc) washing the combined organic layers with saturated sodium bicarbonate 2× and brine 1×. Dry organic with sodium sulfate and concentrate in a large flask under reduced pressure to a dry solid. Dissolve the solid in minimum amount of toluene and add hexane until cloudy. Place mixture in freezer over night. ...
preparation 2
Synthesis of SDS-2 (2S,6S)-2,6-dimethyl-5-methylene-1,3-dioxan-4-one
[0356] The compound prepared in Preparation 1 (13.4 g) was dissolved in 65 mL of dichloromethane (DCM). The resulting solution was cooled to 0° C. using chiller. Methyl acrylate (41.49 mL, 14.5 eq.) and DABCO (2.74 mg, 0.5 eq) was added to the cold mixture. The solution was stirred over the weekend at 0° C. The mixture was concentrated under reduced pressure and purified by flash column chromatography to provide 6 g (86% yield) of the title compound as a colorless oil. 1H NMR(CDCl3) δ 1.48 (m, 6H), 4.64 (m, 1H), 5.50 (m, 1H), 5.59 (d, 1H), 6.49 (d, 1H); and ESI 142.8 (M+1)+, C7H10O3
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight:weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com