Human antibodies that bind human il-12 and methods for producing
a technology of human il-12 and antibodies, which is applied in the field of human antibodies, can solve the problems of not being described, eliciting unwanted immune reactions, and limited use of murine il-12 antibodies in vivo, and achieves the effect of improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Anti-IL-12 Antibodies
A. Screening for IL-12 Binding Antibodies
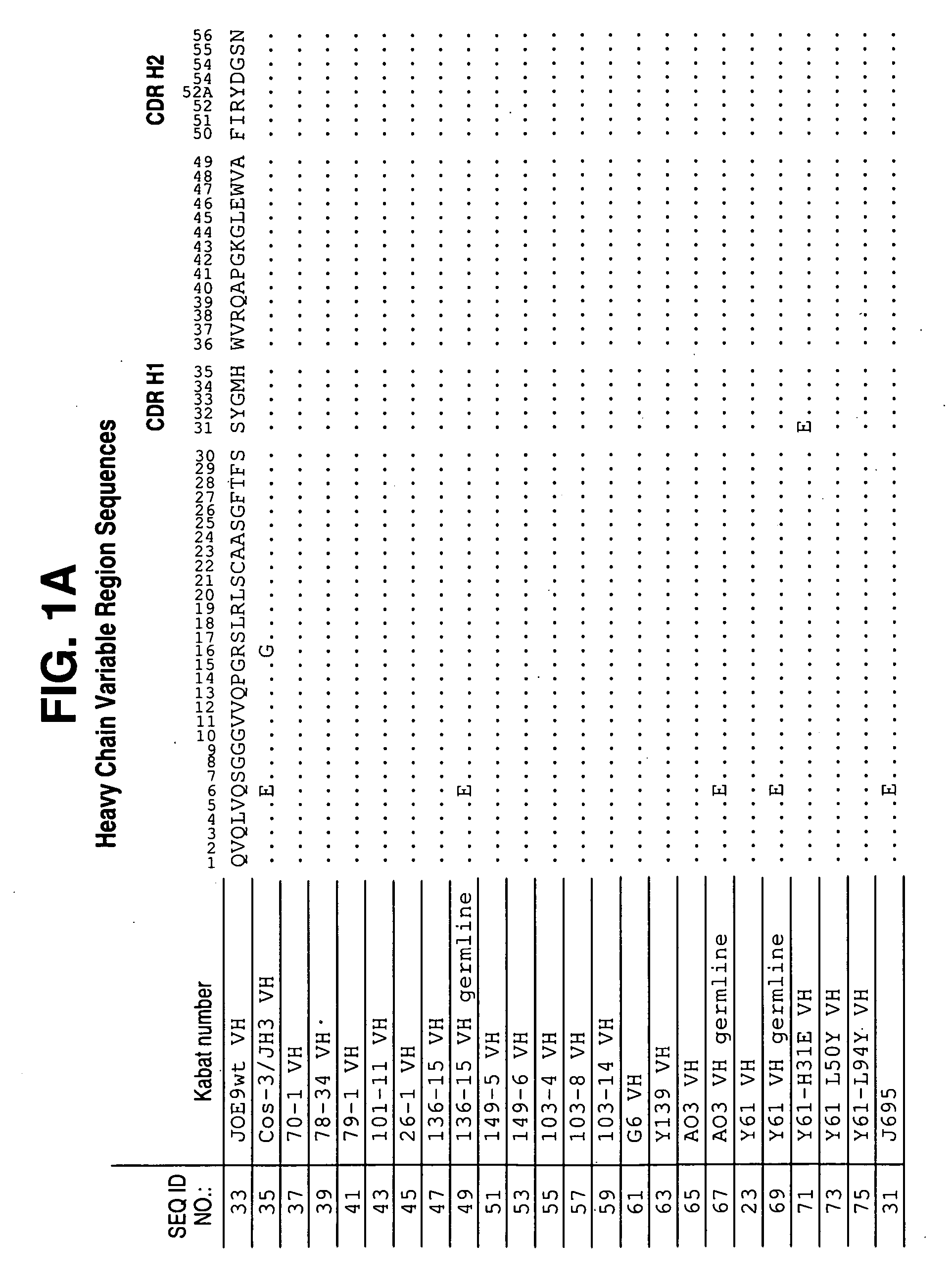
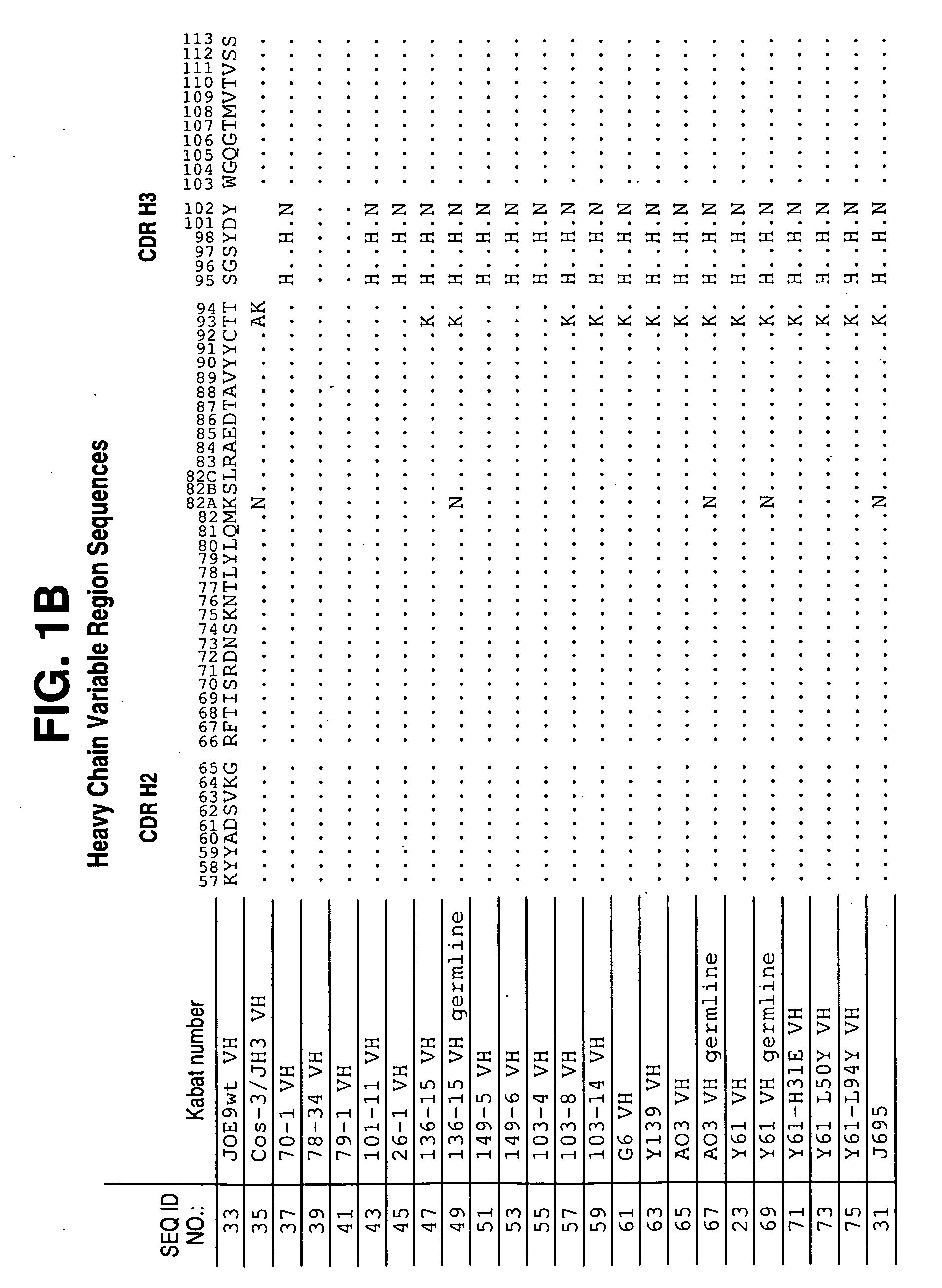
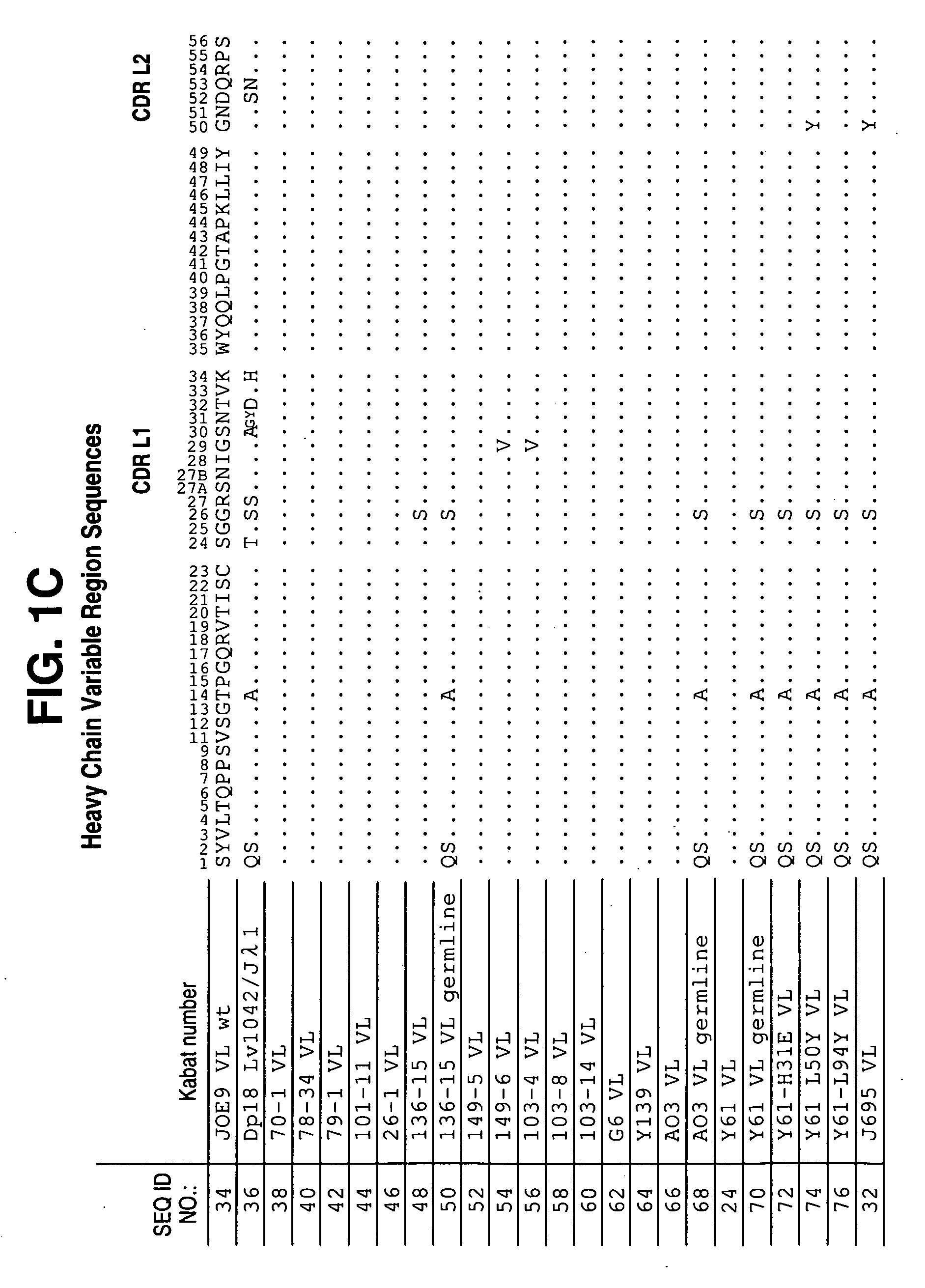
[0615]Antibodies to hIL-12 were isolated by screening three separate scFv phage display libraries prepared using human VL and VH cDNAs from mRNA derived from human tonsils (referred to as scFv 1), tonsil and peripheral blood lymphocytes (PBL) (referred to as scFv 2), and bone marrow-derived lymphocytes (referred to as BMDL). Construction of the library and methods for selection are described in Vaughan et al. (1996) Nature Biotech. 14: 309-314.
[0616]The libraries were screened using the antigens, human IL-12 p70 subunit, human IL-12 p40 subunit, chimaeric IL-12 (mouse p40 / human p35), mouse IL-12, biotinylated human IL-12 and biotinylated chimaeric IL-12. IL-12 specific antibodies were selected by coating the antigen onto immunotubes using standard procedures (Marks et al., (1991) J. Mol. Biol. 222: 581-597). The scFv library 2 was screened using either IL-12, or biotinylated-IL-12, and generated a significant...
example 2
Mutation of Y61 at Hypermutation and Contact Positions
[0631]Typically selection of recombinant antibodies with improved affinities can be carried out using phage display methods. This is accomplished by randomly mutating combinations of CDR residues to generate large libraries containing single-chain antibodies of different sequences. Typically, antibodies with improved affinities are selected based on their ability to reach an equilibrium in an antibody-antigen reaction. However, when Y61 scFV was expressed on phage surface and incubated with IL-12, selection conditions could not be found that would allow the system to reach normal antibody-antigen equilibrium. The scFV-phage remained bound to IL-12, presumably due to a non-specific interaction, since purified Y61 scFv exhibits normal dissociation kinetics. Since the usual methods of phage-display affinity maturation to Y61 (i.e. library generation and selections by mutagenesis of multiple CDR residues) could not be utilized, a new...
example 3
Functional Activity of Anti-hIL-12 Antibodies
[0639]To examine the functional activity of the human anti-human IL-12 antibodies of the invention, the antibodies were used in several assays that measure the ability of an antibody to inhibit IL-12 activity.
A. Preparation of Human PHA-Activated Lymphoblasts
[0640]Human peripheral blood mononuclear cells (PBMC) were isolated from a leukopac collected from a healthy donor by Ficoll-Hypaque gradient centrifugation for 45 minutes at 1500 rpm as described in Current Protocols in Immunology, Unit 7.1. PBMC at the interface of the aqueous blood solution and the lymphocyte separation medium were collected and washed three times with phosphate-buffered saline (PBS) by centrifugation for 15 minutes at 1500 rpm to remove Ficoll-Paque particles.
[0641]The PBMC were then activated to form lymphoblasts as described in Current Protocols in Immunology, Unit 6.16. The washed PBMC were resuspended at 0.5−1×106 cells / ml in RPMI complete medium (RPMI 1640 me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| autoimmune disorder | aaaaa | aaaaa |
| surface plasmon resonance | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com