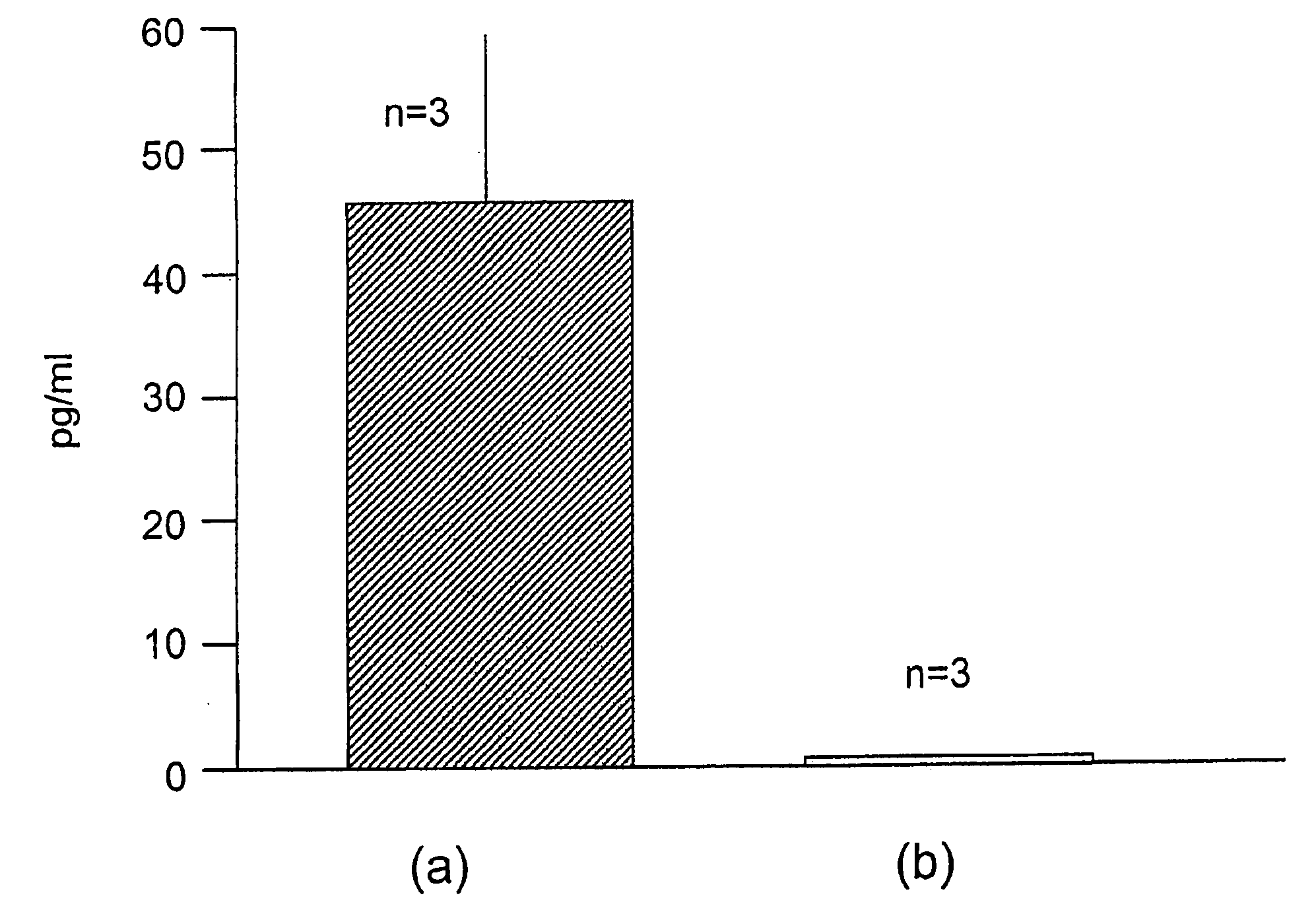
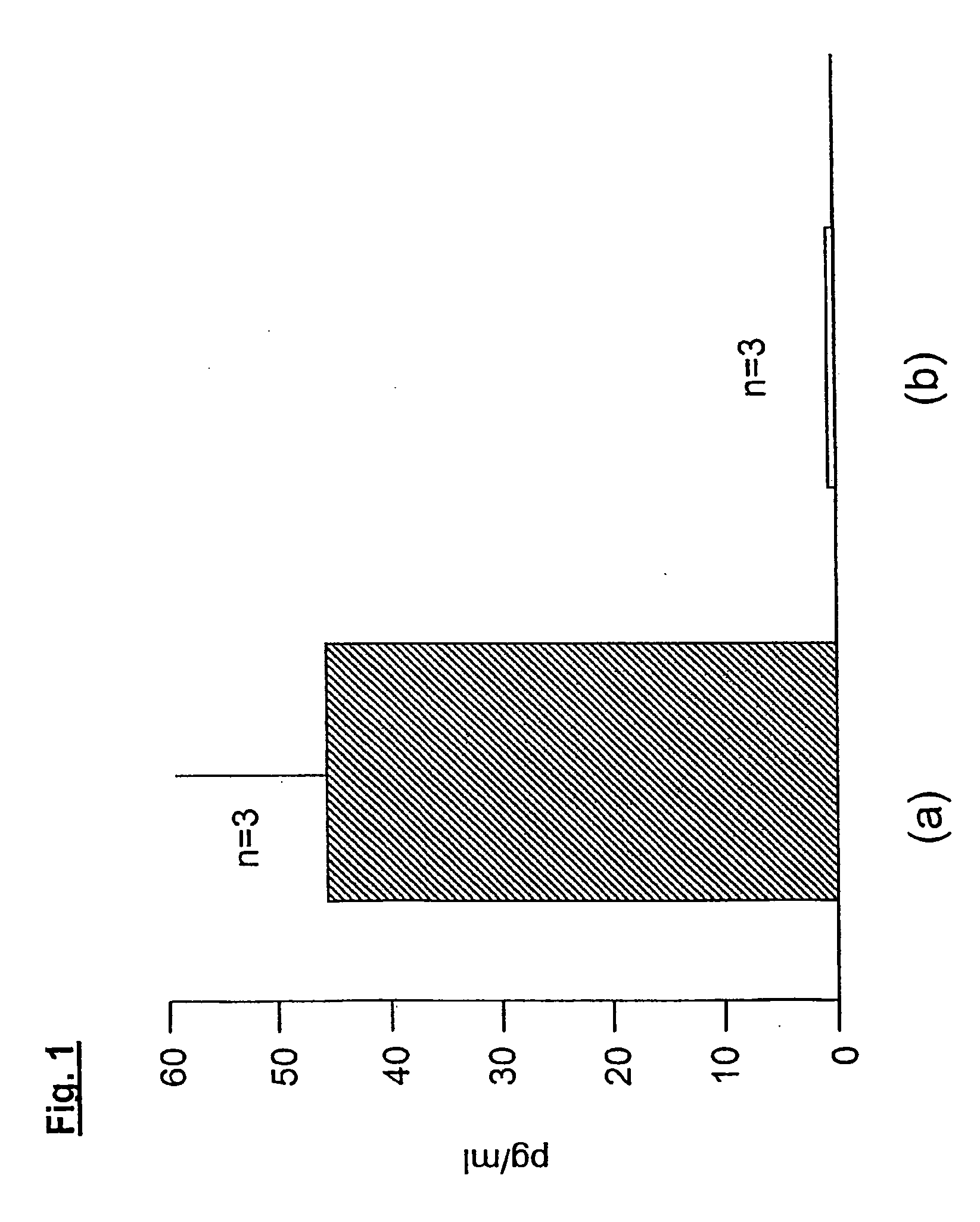
Compounds with the biological activity of vasoactive intestinal peptide for the treatment of pulmonary and arteriolar hypertension
a vasoactive intestinal peptide and vasoactive technology, applied in the direction of peptide/protein ingredients, extracellular fluid disorder, metabolic disorder, etc., can solve the problem of unsatisfactory pulmonary hypertension treatment, and achieve the effect of prevention and/or treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0076]A patient with severe PPH was under therapy with diltiazem, furosemid and an anticoagulant. Right heart catheterisation (Swan-Ganz, Baxter, Irvine, Calif., USA) was performed to measure mean pulmonary artery pressure (mpap), cardiac output (CO), mean arterial pressure (MAP), pulmonary capillary wedge pressure (PCWP) mixed venous oxygen saturation (SvO2%) and systemic arterial oxygen pressure (PaO2%). VIP (100 μg in 3 ml NaCl 0.9%) was inhaled for 15 minutes via the MicroDrop Master Jet (MPV, Truma, Germany) using a particle size of 3 μm to provide alveolar deposition of the substance. Alternatively VIP was injected i.v. 20 (ng / kg.b.w. / min) via portable pump system (CADD-1, Pharmacia-Upjohn, Vienna, Austria). Pulmonary homodynamic and gas exchange were measured before and 15 minutes after inhalation or i.v. injection of VIP. Right heart catheterisation was performed in the intensive care unit. The patient was monitored on-line electrocardiographically, invasive blood pressure a...
example 2
[0077]Increased doses of inhaled VIP in a patient suffering from PPH dose-dependently decrease mean pulmonary artery pressure (mPAP) showing maximum efficacy at a dose of 100 μg.
example 3
[0078]100 μg of inhaled PACAP time-dependently decrease mean pulmonary artery pressure (mPAP) in a patient with PPH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com