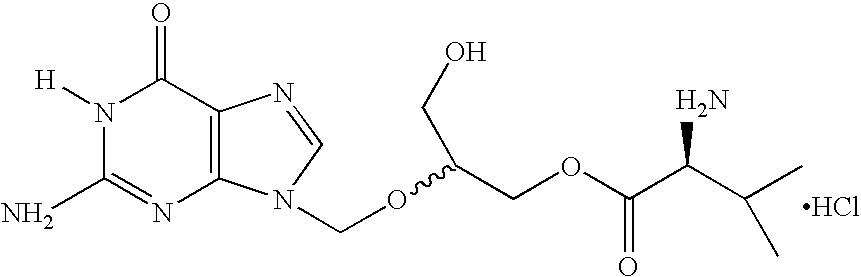
Novel pharmaceutical dosage forms comprising valganciclovir hydrochloride
a technology of valganciclovir and hydrochloride, which is applied in the direction of biocide, animal repellents, dispersion delivery, etc., can solve the problem that the liquid dosage form is unstable for the anticipated shelf life of the produ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0042]A comparison of valganciclovir hydrochloride formulations of Type I and Type II is set out below in Table 2.
TABLE 2Powder for Oral Solution - Comparison of FormulationsType Img / 250 mgType II(constituted =mg / 120 mgIngredients1 mL)(constituted = 1 mL)Formulation NumberJ05F01-03F01-02Valganciclovir55.15155.15155.151HydrochlorideCitric Acid Anhydrous9.50——Sodium Citrate0.40——Sodium Benzoate1.001.001.00Fumaric Acid—2.002.00Povidone K30—2.002.00Sodium Saccharin0.250.250.25Strawberry Flavor5.00——#E187196Tutti-Frutti Flavor—1.801.80#11900-31Maltose, Crystalline178.70——Mannitol—57.8057.80Purified Water—22Total weight per mL250.00mg120.00mg120.00mgBottle Fill Weight15.00g14.40g12.00gAmount of water to be51mL109mL91mLaddedTotal Constituted Volume60mL120mL100mLBottle: Type I amber glass120mL120mL120mL1Equivalent to 50 mg of valganciclovir (as free base) on a dry basis2Removed during processing
Type I Formulations
[0043]The following excipients were used to prepare the formulations of Type I...
example 2
Type II Formulations
[0045]The reason for changing the formulation from Type I to Type II and within Type II was to improve the stability profile of the solid pharmaceutical dosage form and the constituted liquid pharmaceutical dosage form for oral administration. The differences between the Type I and the Type II formulations are set out below.
[0046]Degradation was observed in the Type I formulation and was attributed to valganciclovir hydrochloride / citric acid interaction in the solid pharmaceutical dosage form. Citric acid is a hygroscopic organic acid and appears to degrade valganciclovir hydrochloride in the solid dosage form. Fumaric acid, a less hygroscopic organic acid, was therefore selected to replace citric acid / sodium citrate in the Type II formulations.
[0047]Degradation was also observed in the Type I formulation and was attributed to valganciclovir hydrochloride / maltose interaction in the constituted liquid pharmaceutical dosage form for oral administration. Maltose app...
example 3
Manufacturing Process for Type II Formulations
[0048]The batch manufactured for the clinical Type I formulation was initially based on a dry powder mixture. Upon reformulation with different excipients, the flow properties of the final powder mix were found to be insufficient for appropriate performance. By using wet granulation, the flow properties of the final powder were considerably improved. Since commercial valganciclovir 450 mg tablets utilize an aqueous wet granulation process with povidone K30 as the binder, this process served as the basis for the preparation of the solid pharmaceutical dosage form for constitution with a predetermined amount of water.
[0049]In the present process, the active substance is preblended with povidone K30, fumaric acid, and mannitol. Sodium benzoate and sodium saccharin were dissolved in purified water which served as the granulation solution. The granulation is prepared in a high shear mixer. The flavor is added to the dried and milled granulate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com