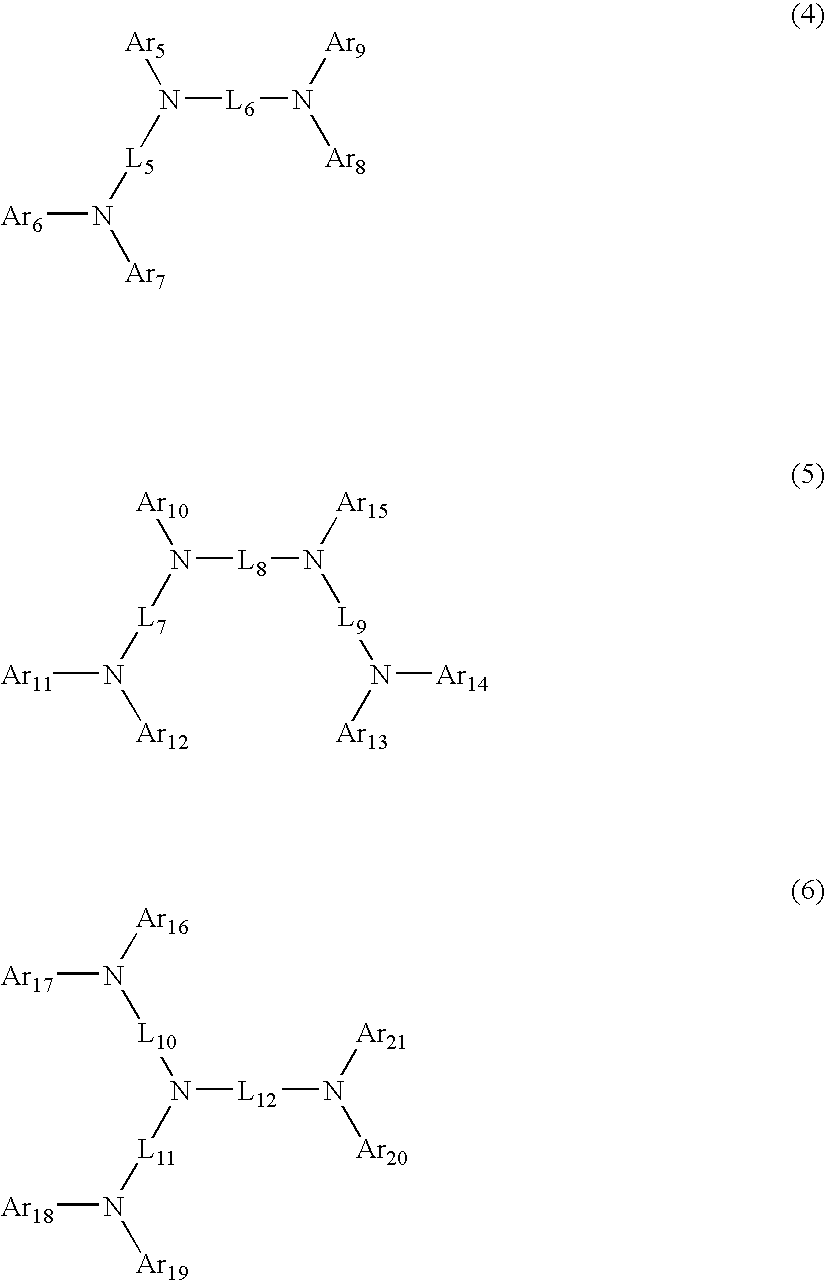
Aromatic amine derivatives and organic electroluminescent device using same
an organic electroluminescent device and amine technology, applied in the direction of luminescent compositions, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of reducing a current efficiency, increasing an operating voltage, and changing the color of emitted, so as to improve the yield of organic el devices and prolong the life. , the effect of less liabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthetic example 1
Synthesis of Intermediate 1
[0178] A three neck flask of 1000 mL was charged with 47 g of 4-bromobiphenyl, 23 g of iodine, 9.4 g of periodic acid dihydrate, 42 ml of water, 360 mL of acetic acid and 11 mL of sulfuric acid under argon flow, and the mixture was stirred at 65° C. for 30 minutes and then reacted at 90° C. for 6 hours. The reaction product was poured into ice and water and filtered. The filtered matter was washed with water and then with methanol, whereby 67 g of a white powder was obtained. The principal peaks of m / z=358 and 360 versus C12H15BrI=359 were obtained by analysis of FD-MS (field desorption mass spectrum), and therefore it was identified as Intermediate 1.
synthetic example 2
Synthesis of Intermediate 2
[0179] A three neck flask of 300 mL was charged with 10 g of p-terphenyl, 12 g of iodine, 4.9 g of periodic acid dihydrate, 20 mL of water, 170 mL of acetic acid and 22 mL of sulfuric acid under argon flow, and the mixture was stirred at 65° C. for 30 minutes and then reacted at 90° C. for 6 hours. The reaction product was poured into ice and water and filtered. The filtered matter was washed with water and then with methanol, whereby 18 g of a white powder was obtained. The principal peak of m / z=482 versus C18H12I2=482 was obtained by analysis of FD-MS, and therefore it was identified as Intermediate 2.
synthetic example 3
Synthesis of Intermediate 3
[0180] A reaction vessel of 50 L was charged with 750 g of phenylboronic acid, 1000 g of 2-bromothiophene, 142 g of tetrakis(triphenylphosphine)palladium (Pd(PPh3)4), 9 L of a 2 M solution of sodium carbonate (Na2CO3) and 15 L of dimethoxyethane under argon flow, and then they were reacted at 80° C. for 8 hours. The reaction solution was extracted with toluene / water, and the extract was dried on anhydrous sodium sulfate. This was concentrated under reduced pressure, and a crude product obtained was refined through a column, whereby 786 g of a white powder was obtained.
[0181] A reaction vessel of 20 L was charged with 786 g of the compound obtained above and 8 L of DMF (dimethylforamide) under argon flow, and then 960 g of NBS (N-bromosuccinimide) was slowly added thereto to carry out reaction at room temperature for 12 hours. The reaction solution was extracted with hexane / water, and the extract was dried on anhydrous sodium sulfate. This was concentrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reduction potential | aaaaa | aaaaa |
| reduction potential | aaaaa | aaaaa |
| light transmittance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com