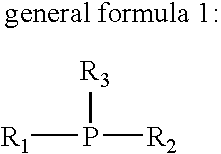
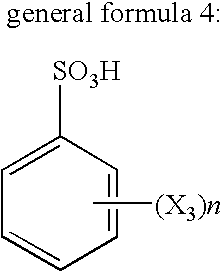
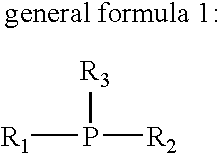
Flux for soldering and circuit board
a circuit board and soldering technology, applied in the field of flux, can solve the problems of affecting the electrical insulation properties between the lands, affecting the bonding strength of soldering, and liable to liberate and deposit independently of silver or copper, so as to suppress the diffusion of nickel, improve the bonding strength of soldering, and suppress the reduction deposition of silver and/or copper
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[Silver or Copper Compound]
[0066] As silver or copper compounds, the following materials were selected. [0067] A: [Ag{P(C6H5)3}4]+CH3SO3−[0068] B: [Ag]+C6H4N3−[0069] C: [Ag]+C2H2N3S−[0070] D: [Cu{P(C6H5)3}3]+CH3SO3−[0071] E: [Cu]+C6H4N3−[0072] F: [Cu]+C2H2N3S−
experimental example 1
[0077] The respective fluxes in Table 1 were printed overall and in a thickness of 100 μm on a tandem-pattern substrate, and heated by using reflow profile whose maximum temperature was 250° C. The substrate was then immersed in an ultrasonic washer filled with butyl carbitol solution of 60° C., to remove the flux. Then, insulating resistance was measured after leaving it for 168 hours in an atmosphere having a temperature of 85° C. and a relative humidity of 85%, while applying a voltage of DC50V to the substrate (Based on the test method of JIS Z3197).
experimental example 2
[0078] Lands were formed on a substrate, which were 0.4 mm in diameter and had nickel electroless plating to copper and further had gold flash plating thereon, and the respective fluxes in Table 1 were printed overall and in a thickness of 100 μm on the substrate. Then, solder balls of tin-37 lead having a diameter of 0.4 mm were placed on the lands, and allowed to reflow at 220° C. so as to connect the solder balls to the lands. Subsequently, the substrate was immersed in an ultrasonic washer filled with butyl carbitol solution of 60° C., to remove the flux.
[0079] Thereafter, with regard to resist portions in the vicinity of en electrode pattern, elemental analysis with an energy dispersive X-ray fluorescence analyzer (EDX) was conducted to examine the presence or absence of the reduction deposition of the metal of silver or copper.
[0080] In addition, with regard to the soldered portions of the soldered substrate, tension test was conducted to measure bonding strength (DAGE-SERIE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com