Method for preparing colloidal solution and carrier having colloidal particles fixed on surface thereof, fuel cell cathode, fuel cell anode and method for preparing the same and fuel cell using the same, and low temperature oxidation catalyst, method for preparing the same and fuel cell fuel modifying device using the same
a technology of colloidal solution and carrier, which is applied in the direction of catalyst activation/preparation, chemical/physical processes, and methods for preparing the same and fuel cells using the same, can solve the problems of fine metal particles that cannot be well fixed on the surface of glass rivers, fine metal particles obtained using a dispersion method have large average particle diameters, and cannot be easily and strongly fixed , to achieve the effect of high catalytic activity, high uniformity and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0044] First, a colloidal solution was prepared according to the description of the above-described publication. Referring to the colloidal solution of Reference Example 1, examples, the reference example and comparative examples will be sequentially described. A 1,500-ml flask, a 100-ml conical flask a 200-ml conical fask a reflux condenser and a stirrer were immersed in aqua regia for a day and night, and the apparatuses were well cleaned using pure water ion-exchanged and ultra-filtered Into the 1,500 ml flask 850 ml of pure water ion-exchanged and ultra-filtered and the stirrer are charged, the reflux condenser is installed on the flask and the pure water was heated to a temperature of 100° C. In order to remove dissolved oxygen, the pure water was boiled as it was for one hour. On the other hand, 0.1328 g of tetratachloroplanic acid, hexahydrate (50 mg as platinum) was weighed and charged into the 100-ml conical flask and pure water ion-exchanged and ultra-filtered was added to...
example 8
[0061] A 1,500-ml flask, a 100-ml conical flask, a 200-ml conical flask, a reflux condenser and a stirrer were immersed in aqua regia for a day and night, and the apparatuses were well cleaned using pure water ion-exchanged and ultra-filtered. Into the 1,500-ml flask, 850 ml of pure water ion-exchanged and ultra-filtered and the stirrer were charged, the reflux condenser is installed on the flask, and the pure water was heated to a temperature of 100° C. In order to remove dissolved oxygen, the pure water was boiled as it was for one hour. On the other hand, 0.1328 g of tetratachloroplanic acid, hexahydrate (50 mg as platinum) was weighed and charged into the 100 ml conical and pure water ion-exchanged and ultra-fitted was added to make 50 ml. One grain of sodium citrate was weighed and charged in the 200 ml conical flask, and pure water ion-exchanged and ultra-filtered was added to make 100 ml. After removing dissolved oxygen of the pure water The aqueous solution of tetratachlorop...
example 9
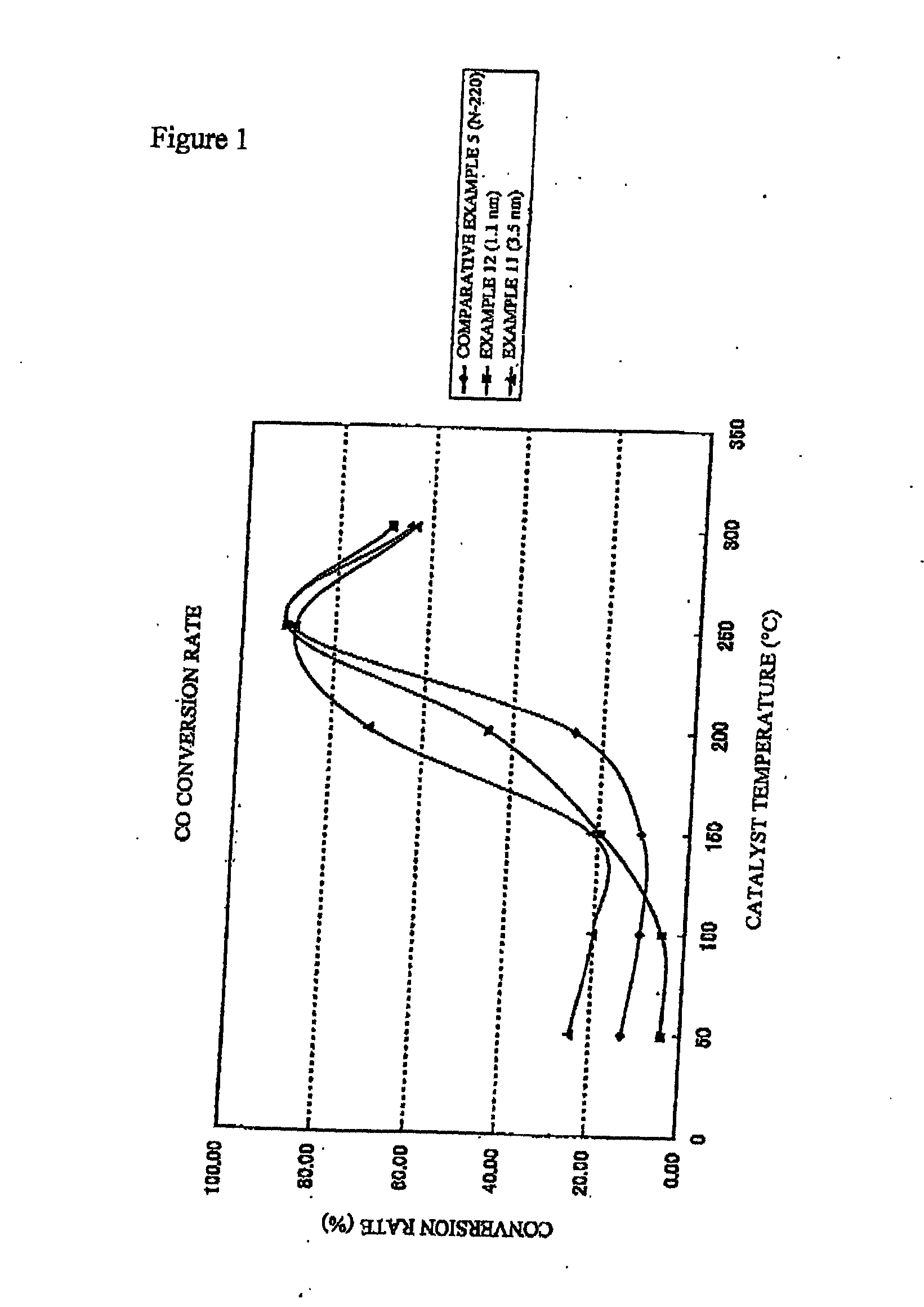
[0064] A colloidal solution and a fuel cell cathode were prepared in the same manner as in Example 8, except that the time from the start of the reaction to the stop of the reaction in the state wherein the aqueous solution of sodium citrate had been completely added in the 1,500 ml flask, and the reducing reaction was continued in the boiling state. The average particle diameter of the colloidal particles of platinum in the colloidal solution measured using a transmission electron microscope was known to be 1.1 nm. The quantity of the colloidal particles of platinum fixed on the surface of the graphite electrode was 10 μg-Pt / cm2 same as in Example 8. The cathode current value measured under the same conditions as in Example 8 was i (O2)=−235 A / g-Pt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com