Agent improving proton-driven transporter-mediated absorption in digestive tract and process for producing the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
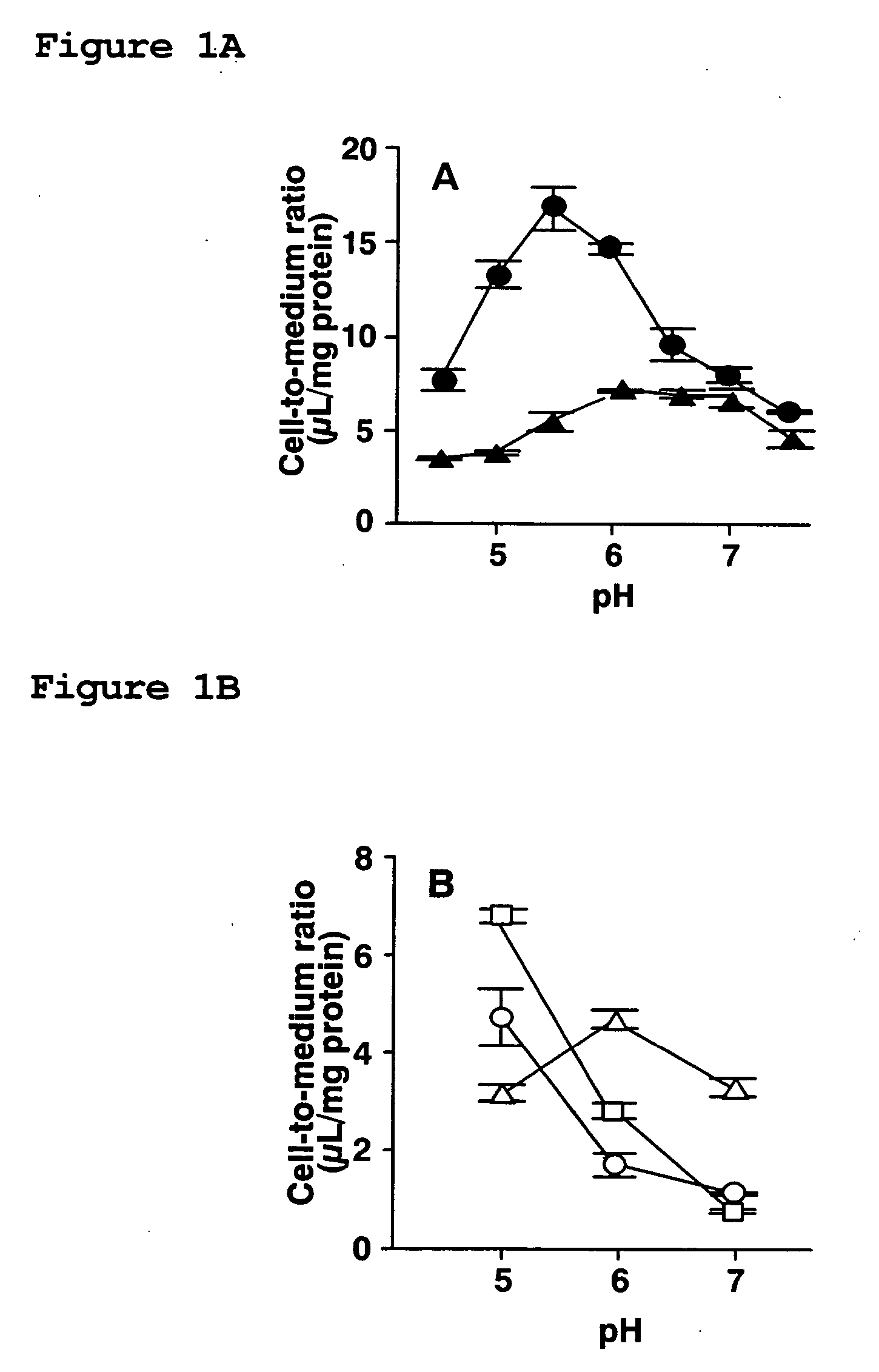
[0094] To investigate the effect of extracellular fluid on the cellular uptake of dipeptides and β-lactam antibiotics transported by PEPT1, the cellular uptake of dipeptides, i.e., [14C]glycylsarcosine ([14C]GlySar) and [3H]carnosine, and β-lactam antibiotics, i.e., cefadroxil (CDX), cefixime (CFIX), and FK089, was evaluated at pH 5.0 to 7.0 using gastrointestinal tract model cells (Caco-2 cells).
[0095] Cells cultured on 4-well plates were rinsed 3 times with 1 mL Hanks' balanced salt solution (HBSS: 0.952 mM CaCl2, 5.36 mM KCl, 0.441 mM KH2PO4, 0.812 mM MgSO4, 136.7 mM NaCl, 0.385 mM Na2HPO4, 25 mM D-glucose, 10 mM HEPES; pH 7.4; osmotic pressure 315 mOs / kg) heated to 37° C., and uptake was initiated by adding 250 μL HBSS containing medical fluid. Uptake was terminated at a predetermined period of time by washing the cells 3 times with 1 mL ice-cooled HBSS. After the completion of uptake, 0.25 mL 5 N NaOH was added, and the cells were agitated for 2 hours to solubilize, followed b...
example 2
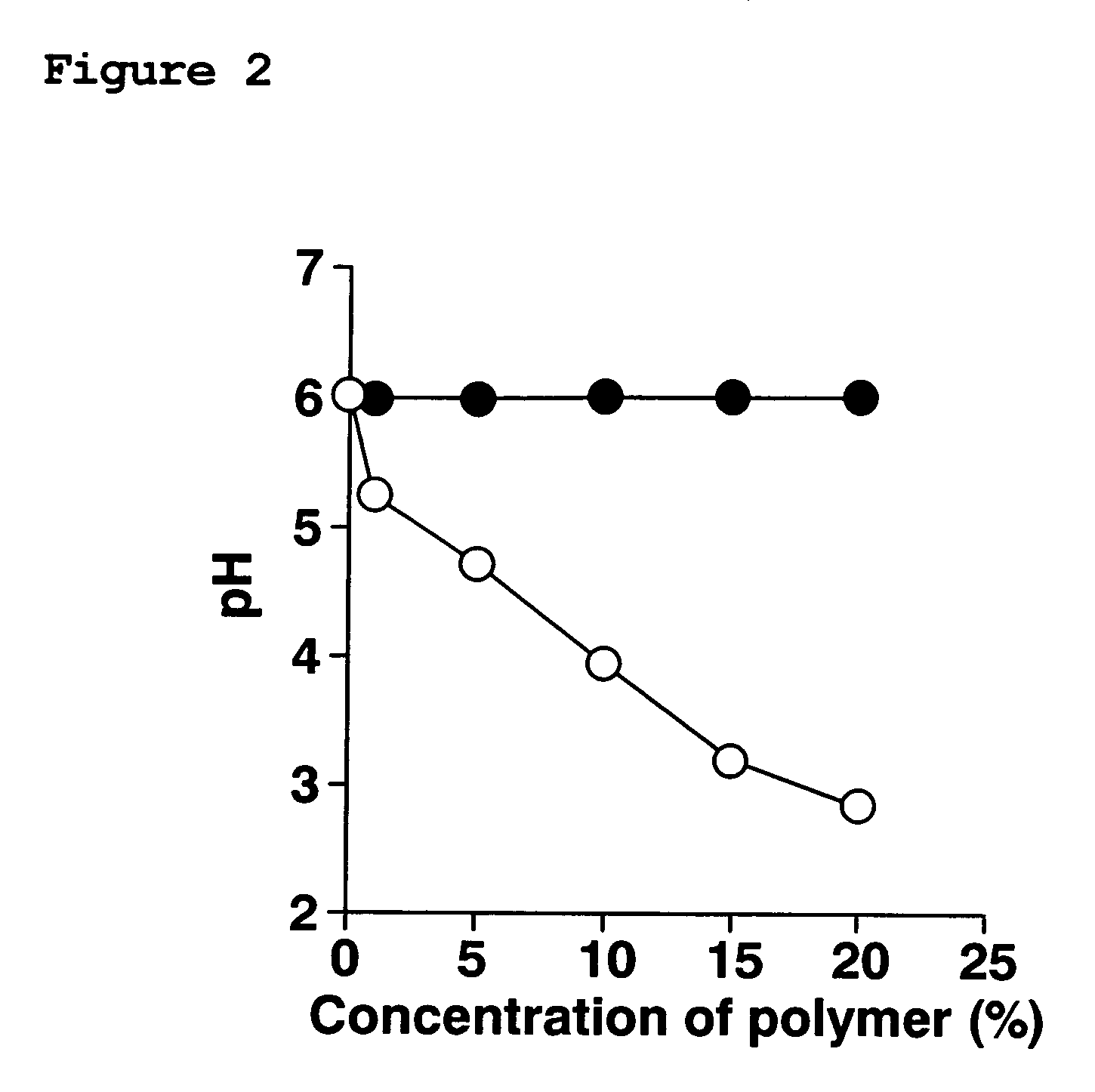
[0101] To investigate whether it is possible to control pH by adding pH-sensitive polymers, the effect of such a polymer on the pH of MES buffer was examined.
[0102] A methacrylic acid copolymer (Eudragit L100-55) was used as a pH-sensitive polymer, and an aminoalkyl / methacrylate copolymer (Eudragit RS PO) was used as a pH-insensitive polymer.
[0103] A polymer (methacrylic acid copolymer Eudragit L100-55 or, of a pH-insensitive polymer, aminoalkyl / methacrylate copolymer Eudragit RS PO) was added to MES buffer (pH 6.0). Subsequently, the pH of the buffer was measured by a pH meter.
[0104] The pH of the buffer decreased as Eudragit L100-55 was added (FIG. 2). In FIG. 2, plots (●) represent the pH of the MES buffer containing Eudragit RS PO, and plots (◯) represent the pH of the MES buffer containing Eudragit L100-55. Compared with the pH of the buffer having no polymer content, pH was decreased to about 3.0 when Eudragit L100-55 was added to a proportion of 20%. In contrast, the use o...
example 3
[0105] To investigate whether gastrointestinal absorption in rats of β-lactam antibiotics under physiological conditions can be improved by controlling the gastrointestinal pH, absorption of a zwitterionic compound (CDX) and an anionic compound (CFIX) in the presence and absence of a pH-sensitive polymer (Eudragit L100-55) was examined using the in situ closed loop method (FIG. 6 shows a diagram).
[0106] The oral composition of the invention was prepared by adding a β-lactam antibiotic (CDX or CFIX) to 10 mM MES buffer (pH 6.0) such that the buffer contained CDX in an amount of 1 mM or CFIX in an amount of 0.5 mM, and further adding Eudragit L100-55 to the buffer so that it contained in a proportion of 10 or 20 wt. % based on the amount of the entire oral composition. A β-lactam antibiotic solution containing no Eudragit L100-55 was prepared as a control.
[0107] These compositions were administered into the intestinal loops prepared at the caecum junction of SD male rats (junction b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com