Particulate composition
a technology of composition and particles, applied in the field of particles composition, can solve problems such as adversely affecting the stability of medicaments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
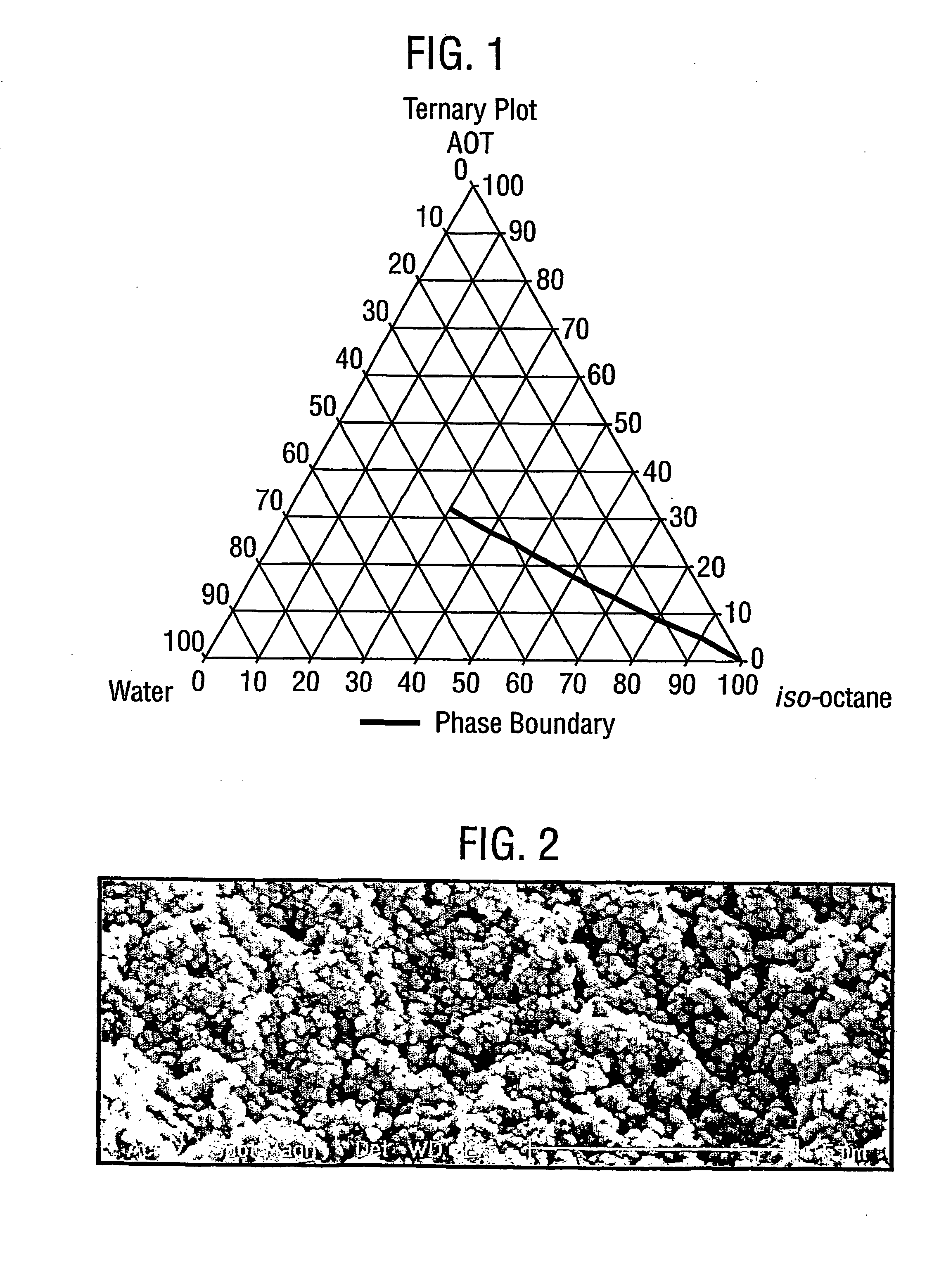
[0109] A microemulsion composition was prepared following the above procedure from 78% w / w iso-octane, 6% w / w sodium bis (2-ethylhexyl) sulphosuccinate, and 16% w / w of an aqueous phase. The aqueous phase comprised, with respect to the aqueous phase, 0.06% w / w bromothymol blue and 17.17% w / w polyacrylic acid (molecular weight 2000), with the balance being water.
[0110] The size of the resulting particles was measured using the photon correlation spectroscopy technique described above employing the Coulter N4. The particles were found to be 232±58 nm, n=7 mean±sd.
[0111] The particles were also subjected to scanning electron microscopy employing the procedure described above. FIG. 2 is the scanning electron micrograph of the present particles. The particles appear spherical and to have a diameter of approximately 250 nm, which is in line with the data from the photon correlation spectroscopy sizing data.
example 2
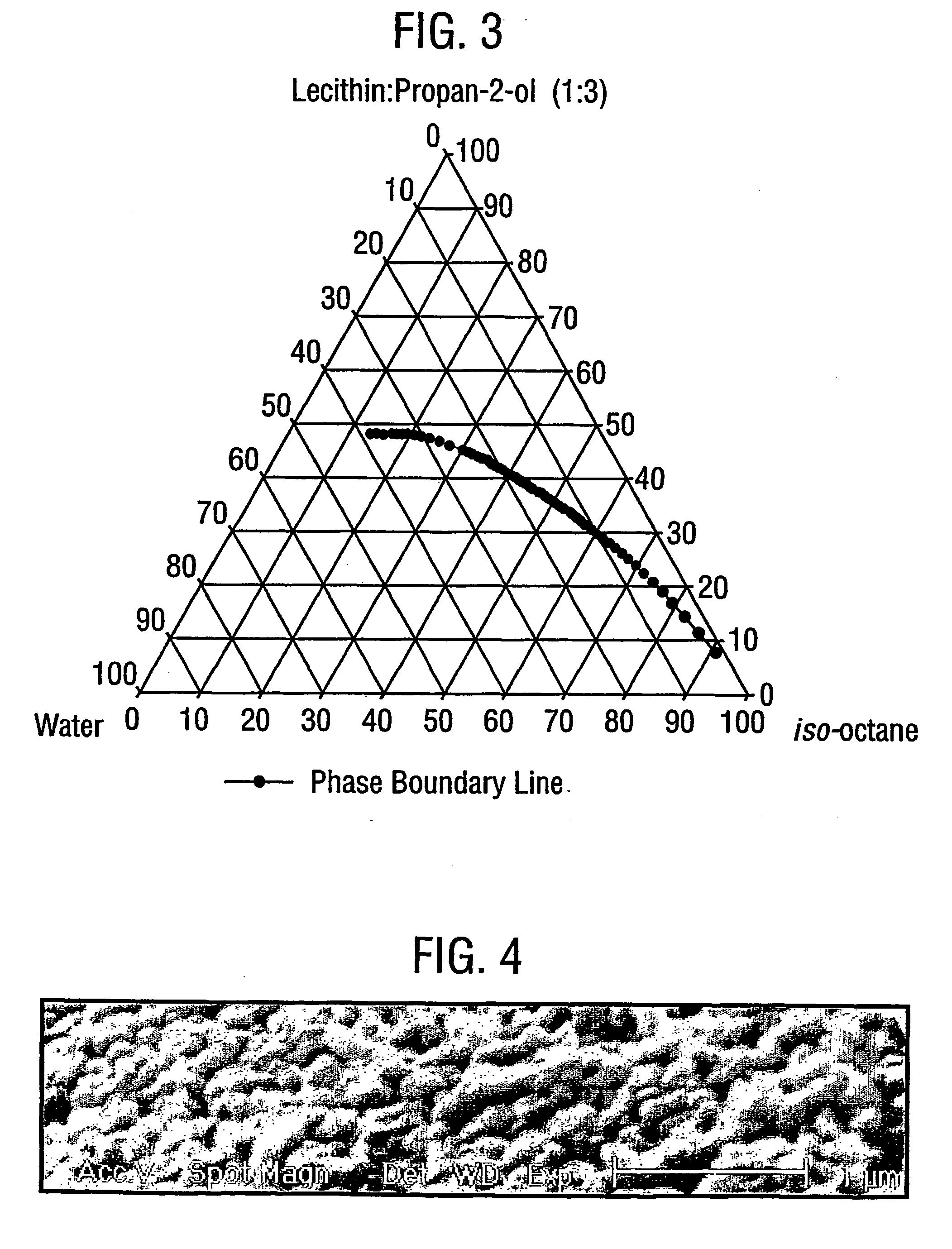
[0112] Microemulsion compositions were prepared following the above procedure for three compositions comprising iso-octane, lecithin:propan-2-ol(1:3 w / w) and an aqueous phase. The relative proportions of each phase and the content of the aqueous phase are given in Table I below.
TABLE ISurfactant (% w / w)Iso-octaneLecithin:Aqueous PhaseFormulation(% w / w)Propan-2-ol (1:3)(% w / w) / compositionB254530 / salbutamol sulphate(17% w / v)C354520 / salbutamol sulphate(17% w / v)D404020 / salbutamolsulphate:lactose(70:30)(17% w / v)
[0113] The size of the resulting particles formed from each formulation was measured by photon correlation spectroscopy employing the Coulter N4 machine in the above described procedure. The results are given in Table II below.
TABLE IINanoparticle size by PCS (scattering intensity)Formulation(mean ± sd, n = 7)BPopulation 1 (50.4%): 216 ± 43 nmPopulation 2 (49.6%): 40 ± 15 nmC34 ± 17 nmD69 ± 39 nm
[0114] A scanning electron micrograph of the salbutamol sulphate nanoparticles res...
example 3
[0115] Each of the nanoparticles resulting from the ternary formulations of Example 2 was employed to produce an aerosol composition.
[0116] In each case ˜30 mg of nanoparticles were dispersed in 0.5 g n-hexane with 3 minutes sonication in a plastic vial for use in a pressurised metered dose inhaler. A Bespak BK 357 100 μl metering valve was crimped in place and hydrofluoroalkane propellant (14 g HFA-227) was added using a pressure burette. The valve was actuated through a mouthpiece with a 0.25 mm orifice.
[0117] Visual evaluation of the compositions was performed prior and subsequent to sonication and at later time points to assess formulation stability and homogeneity.
[0118] The aerosol performance of each resulting nanoparticle HEFA formulations was assessed by cascade impaction. The plates of an Andersen cascade impactor were coated with polyethylene glycol (molecular weight 300) to reduce particle bounce and re-entrainment.
[0119] In each case the pressurised metered dose inh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com